
tshirtdr1 - 19-7-2017 at 11:55
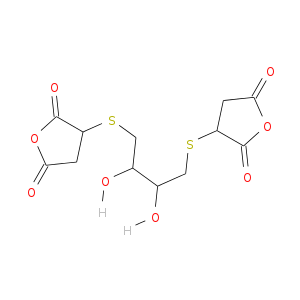
I have been trying to synthesize the following molecule for the past couple of days, but today I considered that it might not be possible. I am
performing this synthesis simply to characterize the sulfur-carbon linkages spectroscopically for future reference. I can't find any references in
the literature for this molecule. I am in an academic lab with relatively good equipment (but not the best).
It looks complex, but if you observe the parts, it is just DTT (Dithiothreitol) and maleic anhydride. (Both are solids.)
I tried two techniques:
1. Using maleic acid and DTT in pH10 buffer to deprotonate the DTT and perform a conjugate addition at the double bond. After the reaction, I tried to
remove the buffer with nitrogen gas, but the solubility of the observed product was high so I heated it. Product decomposed upon heating. Verified
decomposition by NMR.
2. Using Maleic anhydride with DTT in dry diethyl ether over sodium carbonate. Interestingly, with the sodium carbonate, I observed a pink product
briefly (which quickly decomposed) upon decanting the ether from it. Didn't bother with NMR. Got an oily yellow liquid.
I did not try this in an inert atmosphere, but plan to repeat in that perhaps tomorrow. I am thinking I may get side products with the alcohol groups
and the anhydride (or acid). Any opinions? My graduate program was computational, so I am pretty much self-taught at synthesis. I may be missing
something really stupid and obvious.
Thanks.
subskune - 20-7-2017 at 01:00
It seems that you want to add a thiol to a double bond. If it was an alcohol you would add sulfuric acid as catalyst in waterfree solvent.
http://www.kshitij-iitjee.com/Addition-of-Water-and-Addition...
Maybe this works for thiols as well. You might need to protect the alcohol groups first.
Beside from this there are a lot of other mechanisms. On wiki you might get an overview
https://en.wikipedia.org/wiki/Thiol-ene_reaction
Check this for details:
http://www.organic-chemistry.org/synthesis/C1S/sulfides.shtm
Eddygp - 20-7-2017 at 03:26
Conjugate addition should be easy, because RSH are soft nucleophiles. The ROH will need protection, or maybe the yield won't be too negatively
affected.
CuReUS - 20-7-2017 at 06:12
http://pubs.acs.org/doi/abs/10.1021/ja01068a022 - Have you seen this ? Its literally the first thing google came up with
tshirtdr1 - 20-7-2017 at 08:21
CuReUS, Thanks. I am not a current ACS member, and don't have access to the journal. I may try to get it through the library if I don't work it out.
Thanks.
Thanks all for your input.
boilingstone2 - 20-7-2017 at 08:28
It would be a shame if someone downloaded the paper and uploaded it here
Attachment: The Mechanism of the Base-Catalyzed Addition of Thiols to Maleic Anhydride.pdf (480kB)
This file has been downloaded 481 times
tshirtdr1 - 20-7-2017 at 18:23
Thanks!

