
guy - 21-12-2006 at 01:01
Aside from all the inorganic stuff, what organic compounds can I make with sulfur? Any synthesis that uses pretty common organic compounds?
not_important - 21-12-2006 at 02:21
Not too much directly.
acetylene and sulfur over hot pyrite at ... can't remember, several hundred C, gives thiophene, a bit of thiophthene, some higher homologs, and other
stuff.
Styrene and sulfur through a iron tube at 600 C gives benzothiophene
Alkali sulfides and hydrosulfides are reacted with organic halides, tosylates, and so one, to give sulfides (RSR) and mercaptans (RSH).
A solution of polystyrene, to 2% to 10%, in sulfur gives a supercooled material where the sulfur crystallises only very slowly, which is used as an
adhesive and surface coating.
And there's the family of substituted sulfuric acids - the sulfate esters, sulfonic acids, and sulfones; as well as their derivitives. Uses sulffuric
acid, SO3, HSO3Cl, SO2Cl2, and so on, not elemental sulfur.
Heating sulfur with many organics give H2S and less saturated organic, with or without rearrangement depending on the starting structure. Sometimes
sulfur is found combined in the product, depending.
YT2095 - 21-12-2006 at 06:38
Wow, Thnx not_important , I actualy enjoyed that post to 
the only thing I`ve ever done directly with it is dissolve it in hot Toluene and cool Very slowly to see how big I could make a single crystal 
unionised - 21-12-2006 at 07:54
There's the reaction with Grignard reagents too. It gives alkyl sulphides or disulphides.
Not very useful unless you happen to have the Grignard reagent and nothing to do with it.
It's mildly intreesting that you can do the same thing in 2 ways.
You can react the alkyl halide with a metal (eg Li) to get an organometalic then react that with sulphur to get the sulphide or you can react the
metal with sulphur and then react this with the halide to get the sulphide.
(Only works with some metals and I don't think the yeild would be good)
not_important - 21-12-2006 at 08:43
YT2095 - how well did you succeed with the crystal growth?
Hmm - I've net heard of the direct reaction of oranometallics with sulfur; thanks for that. And I forgot to mention disulfides above.
guy - 21-12-2006 at 13:42
Thanks not_important. Ill check those out.
I read ferrous sulfide is a strong reducing agent capable of reducing ketones to thiols (Kaschke et al.,
1994)
[Edited on 12/21/2006 by guy]
Ozone - 21-12-2006 at 16:44
mmm, stinky.
Interesting topic,
Except for the old plastic sulfur trick (or the old "Stromboli"), I think that the first stage would be to burn it. Varying the O2 levels during the
burn can effectively select either SO2 or SO3 formation (the SO3 can, of course, be used to make H2SO4 or to fortify your H2SO4 into oleum). The SO2
is a very effective bleaching agent (reductive bleach) and can add (via water-->sulfurous acid, vis. sulfitation) to carbonyl groups yielding the
organosulfite which is usually water soluble.
Alkanolamines are used to scrub SO2, so some chemical reaction is occuring there.
I suppose it needs some research from here.
YT2095, The crystallization sound cool, that would make a neat paperweight for my desk!
Overall, a ketone-->thiol rxn would be a reduction; this would involve multiple steps including the evolution of a reactive nucleophilic species
(viz., sulfite, or -SH, or with acid, H2S , the attack of the carbonyl to yield
the intermediate thiolalkanolate anion, and subsequent elimination of (probably) water. Guy, do you have the Kaschke paper (or a journal reference)?
, the attack of the carbonyl to yield
the intermediate thiolalkanolate anion, and subsequent elimination of (probably) water. Guy, do you have the Kaschke paper (or a journal reference)?
Keep your clothes-pins handy,
O3
guy - 21-12-2006 at 19:40
No, I found that reference from another article on iron-sulfur world theory. Im still trying to look for that article. Ill post it if I see it.
woelen - 22-12-2006 at 03:29
I also noticed that sulphur reacts with butane gas. I put some sulphur in a test tube and poured some liquid butane in it from a cigarette lighter
pressurized bottle, and stoppered the test tube loosely. Then I let the liquid evaporate (this is easy, as the BP of butane is around 0 C) and as soon
as all liquid is gone, I stoppered the test tube very tightly. In this way, it is assured that no air is in the test tube anymore. Next, I heated the
test tube (while keeping it tightly stoppered) at the place where the sulphur is. The sulphur melts and at a certain point, it reacts, forming some
sticky stuff. After cooling down of the test tube, I opened it and I was greeted with a horrible smell.
If you repeat this experiment, be sure to use a thickwalled tube. Due to heating the pressure will rise in that tube, and it may well go over 2
atmosphere if the temperature goes to 300 C or so.
Maya - 22-12-2006 at 05:27
congrats! you just made H2S, rotten eggs gas smell
Sulfur reacts with parafins ( butane ) to make H2S
woelen - 22-12-2006 at 10:27
Yes, it makes H2S, but it also makes something else. I know the smell of H2S, but the smell from this test tube was MUCH worse and also much more
sticky. I think that the smell was due to a mix of H2S, and some sulphuretted hydrocarbon.
Ozone - 22-12-2006 at 11:33
Does it smell like skunk? Butanethiol is a dead-ringer for skunk. The smaller, "stinkier" things would require either air (which you excluded, COS,
etc.) or fragmentation of the butane (which would be unlikely w/o catalyst). Maybe a multiple thiolation?
Clothespin at the ready,
O3
woelen - 22-12-2006 at 13:53
I don't know the smell of skunk, we don't have that kind of animals over here  .
.
I did my best to exclude air (because of safety reasons, I did not want burning butane gas), but of course, small amounts of air may have entered the
tube, but _if_ some air was there, it only was a very small amount.
I found this reaction between butane and sulphur by accident. I tried to make phosphorus sulfide from a mix of red P and S, and I wanted to use butane
as a protective atmosphere (otherwise the phosphorus catches fire in the air). During that experiment I noticed that sulphur reacts with butane, and I
did a counter experiment with only sulphur.
guy - 22-12-2006 at 14:39
That's odd, how can sulfur react with an alkane?
unionised - 22-12-2006 at 15:01
One plausible reaction would be
C4H10 + S --> CH3CH2CH2CH2SH
That would give the thiol which will stink.
These sort of pyrolysis reactions are often what the textbooks call "mechanistically obscure" ie they don't know how the reaction happens; it's
traditional to blame it all on free radicals.
guy - 22-12-2006 at 17:59
Here is an article on sulfur reacting with alkanes, alkenes, and alkynes.
With alkanes, a thiol is formed.
Attachment: Sulfur and alkanes,alkenes,alkynes.pdf (704kB)
This file has been downloaded 1848 times
Ozone - 22-12-2006 at 19:45
Thanks!
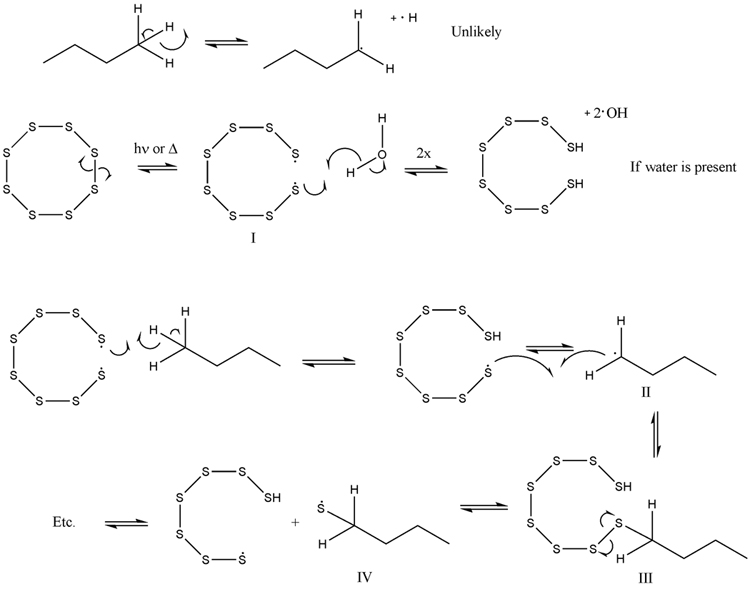
Though the paper cites a theoretical resolution to a mechanism involving, it looks like, insertion of S into a C-H bond, I think that the step-wise
radical mechanism is more feasible (see thiol-ene polymerization and others). Just playing around with the arrows and dots gives (see picture).
The first line is not really that feasible--I suspect any mechanism exploiting hydrogen radical.
The second is realistic as a small amount of water might be present (note, these will all be evanescent species) and likely leads to the third (water
maybe catalytic). Here, the thiol radical abstracts a proton from the butane to yield a 2° radical (which can rearrange to any other equivalent site)
which will immediately react with the S-S or S-H bonds present (see chain transfer).
From here, the S-S bond is much easier to break than the C-S bond so the thiyl radical is expected. This species can then become a thiol via proton
abstraction, terminate with another thiyl radical (this will reinitiate soon) another alkyl radical, or perhaps, disproportionate. Most likely, chain
transfer to S-S or SH will occur as these species should be present in excess.
Just an idea,
O3
[Edited on 23-12-2006 by Ozone]
unionised - 23-12-2006 at 05:06
Come to think of it, once you have hot sulphur at least some of it is in the form of S2 molecules- (especially in the gas phase). Then this reaction
starts to look like oxidation of butane with oxygen (the atom next to sulphur in the periodic table). That reaction has probably been studied in great
detail.
Sauron - 23-12-2006 at 10:13
Sulfur chemists generally only socialize with other sulfur chemists. The stench of many sulfur compounds gets into the clothing, the hair and the skin
and is quite persistent and hard to get rid of.
I must admit to being fascinated with a few sulfur compounds:
1. BEDT-TTF an organic superconductor charge couple complex, see it in Org.Syn.
2. the dithianes and trithianes (1,3-dithiane, 1,4-dithiane, and sym-trithiane) also in Org.Syn. Some of these are useful reagents in Al Meyers'
aldehyde synthesis.
3. An obscure mustard analog called USAF HA-5. This is simply the same same compound as sulfur mustard but the terminal chlorines have been replaced
with nitriles. I'd just like to know what this stuff does: is it a vesicant (by crosslinking DNA and preventing replicating and repair) or antiplant
or ? The prep is in the lit, but I am not interested in making it, just understanding it.
apidej - 3-1-2007 at 08:50
good,thank you



 , the attack of the carbonyl to yield
the intermediate thiolalkanolate anion, and subsequent elimination of (probably) water. Guy, do you have the Kaschke paper (or a journal reference)?
, the attack of the carbonyl to yield
the intermediate thiolalkanolate anion, and subsequent elimination of (probably) water. Guy, do you have the Kaschke paper (or a journal reference)? .
.