
DFliyerz - 10-7-2016 at 12:11
I recently noticed that potassium thiocyanate has a very low melting point when anhydrous; 173.2C. I did some googling and couldn't come up with much,
so I wanted to ask here if electrolysis of molten potassium thiocyanate or perhaps sodium thiocyanate (MP 287C, but decomposes at 307C) could perhaps
result in the formation of the alkali metal.
kmno4 - 11-7-2016 at 03:15
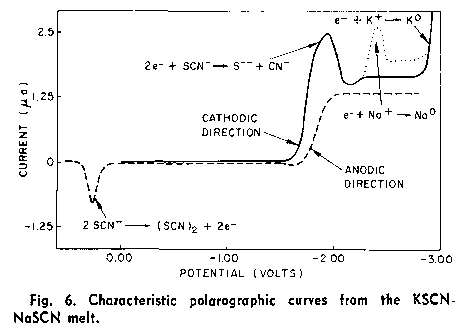
I also did some googling and in 2 minutes (changing keywords) found this: "Advances in Molten Salt Chemistry vol.3" and from it: R. E. Panzer and
M. J. Schaer, J. Electrochem. Soc. 112:1136 (1965).
Shortly: Na(K) reacts with SCN(-) forming S(2-) and CN(-) ions.
In another words, reaction:
Na(K)(+) +e → Na(K)
occurs at more negative potential than reaction:
SCN(-) + 2e → S(2-) + CN(-).
The picture explains everything.
DFliyerz - 11-7-2016 at 09:49
So, it's not possible, or it is possible just under certain conditions?
