
soma - 26-3-2016 at 01:20
In the clemmensen reduction an aldehyde or ketone is reduced using zinc amalgam and hcl in a solvent with heat. I've seen claims that using only zinc
dust with an acid at room temperature stirring for a few hours can reduce oxides although I haven't seen any published papers on this.
Comments?
MeshPL - 26-3-2016 at 04:04
Supposedly it works best with aromatic ketones. But other also work.
Wikipedia mentions zinc powder being preffered to analgam in such cases and provides a reference:http://www.orgsyn.org/demo.aspx?prep=cv6p0289
DJF90 - 26-3-2016 at 04:12
I'm interested to know if other activated forms of zinc could be used, e.g. Zn/Cu couple.
solo - 26-3-2016 at 09:22
Reference Information
THE SYNTHETIC USE OF METALS IN ORGANIC CHEMISTRY
ARTHUR J. HALE,
Zinc and Mercury
Contents
CHAPTER I.
SODIUM AND POTASSIUM.
Synthetic Use of the Metals—Sodium ethylate—Sodamide
—Sodium hydroxide—Potassium cyanide—Potassium
"bisulphate—Potassium hydroxide —Potassium nitrate
—Potassium and sodium disulphates . . . . 1
CHAPTER II.
COPPER AND SILVHXt.
Use of the Metals—Copper acetylene derivatives—Silver
cyanide—Silver hydroxide 35
CHAPTER III.
MAG-NESIUM, CALCIUM AND BARIUM.
Applications of the Grignard reagent—Carbides of Calcium
and Barium—Nitrogen fixation—Calcium and Magnesium
nitrides—Hydroxides of the metals . 42
CHAPTER IV.
ZINO AND MERCURY.
Metallic Zinc—Zinc chloride—Mercuric oxide—Metallic
mercury ^
V1U CONTENTS
CHAPTER V.
ALUMINIUM, TIN AND LEAD.
PAGE
Aluminium chloride—Aluminium-mercury couple—Tin and
lead organo-metallie compounds—Tin tetrachloride—
Lead oxide—Antimony and the chlorides of antimony—
Vanadium pentoxidc 11
CHAPTER VI.
IRON, NICKEL AND PLATINUM.
Ferrous sulphate—Ferrous potassium oxalate—Iron and
ferric chloride—Reduction by reduced iron, nickel or
cobalt 88
APPENDIX I.
PRACTICAL WORK: SODIUM—POTASSIUM.
Ethyl benzene—Anisole—Benzoic anhydride—Hexamethylene—
Trimethylene dicarboxylic acid—Chlorof ormic
ester—Carbonyl chloride—• Ethyl benzoate—Toluic ethyl
ester—Aeetoacetic ester—Ethyl acetoacotic ethyl ester
—Aceto-succinic ester—Malonic ester—Ethyl malonic
ester—Diaceto-succinic ester—Ethane tetra-carboxylic
ester — Acetyl-acetoacetic ester—Antipy rine—Methyl
succinic ester—Succino-succinic ester—Tin tetraphenyl
—Lead tetraphenyl—Mercury diphenyl—Silicon tetraphenyl—
Oxalyl-acetic ester—Hydroxy-methylene camphor—
Acetyl-acetophenone—Ethyl acetophenone—Furfurol
acrolein—Cinnamyl-vinyl-methyl ketone—Acetic
anhydride — Benzoin — Desyl-acetophenone — Phonanthroxylene-
acetoacetic ester 104
APPENDIX II.
PRACTICAL WORK : COPP1R—SILVER.
Acrolein—Acrylic acid—/3-Iodopropionic acid—Adipic acid
—Carbazole—o-Nitrophenyl-propiolic acid—Di-o-nitrophenyl-
diacetylene — o-Chlor -toluene — o-Chlor-benzoio
acid—jp-Chlor-toluene—Formaldehyde . . . . 128
CONTENTS IX
APPENDIX III.
PRACTICAL WORK: MAGNESIUM—CALCIUM.
PAGK
Benzoic acid—Phenyl-ethyl-carbinol—Trimethyl carbinol—
Triphenyl carbinol—Camphoric anhydride—Homo-camphoric
acid—Camphor—Pentamethylene—Cyanamide . 137
APPENDIX IV.
PRACTICAL WORK : ZINC—MERCURY.
Citric acid—Use of Zinc alkyl iodide—Naphthalene—Isoquinoline—
ITuorescein—Malachite green—Acridine—
a-Methyl-indole—Propyl chloride—a-Ethoxy-quinoline
—Phthalic acid 144
APPENDIX V.
PRACTICAL WORK: ALUMINIUM—TIN—LEAD.
Dimethyl-aniline-phosphor-chloride — jp-Tolxiic-aldehyde —
Diphenyl-methane—a-Hydrindone—Triphenyl-methane
—Acetophenone—o-Benzoyl-benzoic acid—Anthraquinone
— Hydrolysis of anisole—Toluene—Diphenyl—
Oxalic acid 153
APPENDIX VI.
PRACTICAL WORK: IRON—NICKEL.
o-Amino-benzaldehyde—o-Amino-cinnamic acid—Mannose
—Hexahydrobenzene—Hexahydrophenol . . .162
INDEX 167
Attachment: Zinc and Mercury from The_Synthetic_Use_of_Metals_in(BookZZ.org)-2.pdf (241kB)
This file has been downloaded 509 times
Attachment: The_Synthetic_Use_of_Metals_in(BookZZ.org).pdf (1.5MB)
This file has been downloaded 587 times
soma - 28-3-2016 at 00:34
Thanks to everyone.
In the article that Solo posted it says that "In effecting the reduction of oxygen compounds
by this method, zinc dust is mixed with the substance
to be reduced, and the mixture is then heated in a
combustion tube. The reduction is often facilitated
by passing a stream of hydrogen or carbon dioxide
through the heated mixture :
By this method succinimide can be converted to
pyrrol".

So this can be used instead of LAH?
soma - 28-3-2016 at 00:57
In the article that MeshPL posted, the zinc was "activated" by stirring with 2% hcl and then washing and drying.
I wonder how much more effective that is than using the unactivated zinc. They claimed to be able to reduce the oxide in ether at -10C temps. This
would be a great improvement over using LAH in THF and raised temps.
[Edited on 28-3-2016 by soma]
Pumukli - 28-3-2016 at 03:51
So this can be used instead of LAH?
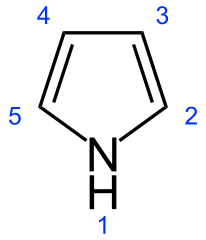
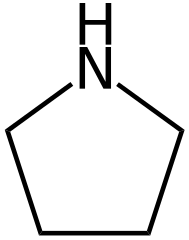
No. What you draw is pirrolidine and not pirrole. Pirrole is an aromatic heterocycle, pirrolidine is alicyclic.
JJay - 28-3-2016 at 07:36
Ohh now I see what is going on here.
Pyrrole:
Pyrrolidine:
[Edited on 28-3-2016 by JJay]
soma - 28-3-2016 at 13:55
Yes. Actually I knew that pyrrole had double bonds but wasn't remembering. The text draws "pyrrol" like this:
CH:CH\
| N
CH:CH/
I didn't notice that the ":" represented double bonds, and left them out.
(edit: for some reason the N isn't coming out in the right place.)
[Edited on 28-3-2016 by soma]
[Edited on 29-3-2016 by soma]
soma - 29-3-2016 at 00:34
In explanations of clemmensens they say that the H from the acid is utilized. I wonder if it's a similar mechanism that makes LAH more active in the
presence of P2O5 or fuming sulfuric.
[Edited on 29-3-2016 by soma]
