
Cesium Fluoride - 26-11-2015 at 15:36
Hi everyone,
What is the lowest reported eutectic for an ice-solid mixture without any co-solvents?
The lowest I know of is the CaCl2-H2O system, which is around -50 C. I vaguely recall reading about lower ones (perhaps with
perchlorates?), but I can't remember where.
Thanks for your help.
P. S. It's been many years since I've been on this site- thanks for all of the great memories.
BromicAcid - 26-11-2015 at 20:04
Instead of simply eutectic, do you mean a frigorific mixture?
Pok - 27-11-2015 at 04:11
This could be the source you remembered (about silver perchlorate): "The eutectic temperature, -58.2°, is lower than that of any other true
salt in water, and only in the cases of hydrogen chloride in water, potassium hydroxide in water, and sulfuric acid in water have lower eutectics been
found. "
Eutectic points (with water) from other sources:
H2SO4: ca. -65 °C
KOH: -65.2 °C
HCl: ca. -86 °C
But more recent literature contradicts this statement, because there are other "true salts" with lower eutectic points than silver perchlorate:
Fe2(SO4)3: -68 °C [source]
Mg(ClO3)2: -69 °C [source]
CaI2: -77 °C
CaBr2: -83 °C [source for both]
Also NH3-H2O has a lower eutectic point (about -98 °C).
Alkaline earth perchlorates:
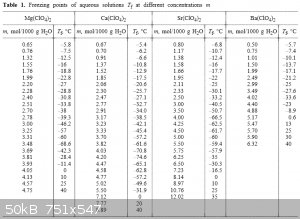
http://link.springer.com/article/10.1007%2Fs11167-005-0306-z
I think CaBr2 is the best candidate if you want a solid/water eutectic.
[Edited on 27-11-2015 by Pok]
User123 - 27-11-2015 at 05:48
Exactly what should be done to get to -50C with calcium chloride and ice? I have tried mixing them and it does not seem to get very much colder than
the ice alone. The ice simply melts. Am I doing something wrong, or SHOULD that be as easy as it is?
Pok - 27-11-2015 at 06:29
You have to use the dry hexahydrate, mix it in the correct proportion (eutectic!) with dry snow or dry and finely ground ice,
insulate the container, precool the ingredients. Every single point is important to get close to -50 °C.
[Edited on 27-11-2015 by Pok]
Metacelsus - 27-11-2015 at 09:20
Yes, don't use the anhydrous salt, as that will produce heat upon hydration.
User123 - 27-11-2015 at 12:23
My method was to dissolve some calcium chloride in cold water, then add lots of ice to it.
Anyway, thanks for the suggestion, I'll try intimately mixing them with a pestle and mortar, and see if I can solidify some appropriate solvents,
since I don't have a sub-zero thermometer.
Any idea on the correct proportion? I did a little searching for correct eutectic proportions, but physical chemistry is not my forte, and it hampered
my searching.
Cesium Fluoride - 27-11-2015 at 21:05
Thanks Pok for the wonderful information. This is exactly what I was looking for and more!
alive&kickin - 3-12-2015 at 07:17
Searching I found this: https://the-hive.archive.erowid.org/forum/showflat.pl?static.... Hope it helps if this is what someone is looking for.
