| Sulfuric acid is quite useful, its used as a acid catalyst in a voluminous amount of reactions because of its properties. Its a strong acid and also
can remove water from many reactions. For example in the synthesis of methyl benzoate were water can drive the reaction back yielding the reactants.
For example nitric acid is not used because it often acts like a base, this is why sulfuric acid reacts with it to form the nitronium ion responsible
for nitration. |


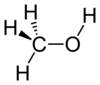 + H2SO4 -->
+ H2SO4 --> + H2O -->
+ H2O -->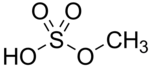 x2
x2 -- same process -->
-- same process --> 


