Originally posted by rogue chemist
H2 and Cl2 is very voilent once initiated. There is a demo which consists of filling a thick glass tube with a stoichiometric mix of the gasses in
the dark, stoppered, and then a 'photo' is taken of the tube when it is in the light and the flash initiates the explosvie reaction blowing out the
stoppers.
Here is Tacho's experiment with something very similar. https://sciencemadness.org/talk/viewthread.php?tid=2154#pid2...
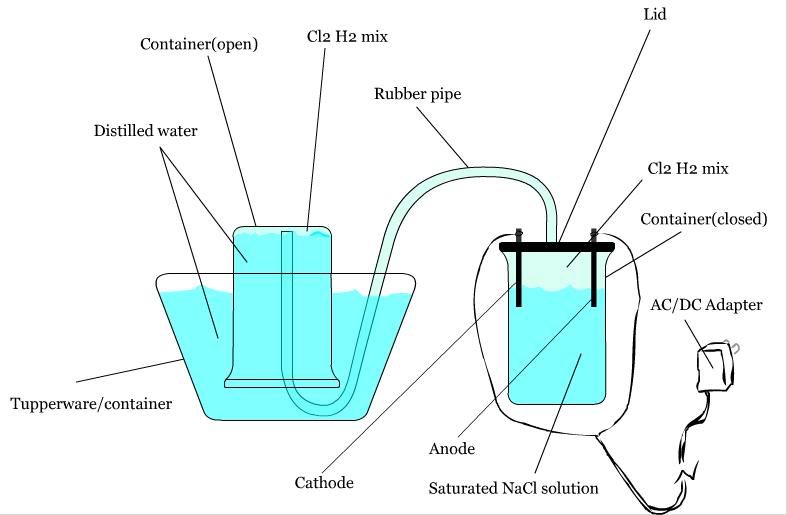
Yes, your method is feasible, but you need some work on how you are going to react the H2 and Cl2.
EDIT: You should use separate tubes for the hydrogen and oxygen coming off the cell, otherwise an explosion could blow your electrolysis cell as well
as your reaction vessel.
[Edited on 22-5-2006 by rogue chemist] |