I wanted to share an experiment that I've been thinking about and see if any of the skilled organic chemists we have here had any advice (especially
regarding the separation of isomers).
Electrophilic Aromatic Substitution of Naphthalene with Iodine Monochloride
Background
The preparation of bromobenzene has been used as a laboratory exercise to demonstrate the principle of electrophilic aromatic substitution. [1]
However, the relative scarcity and toxicity of benzene may make this reaction unattractive to the home chemist. The feasibility of a slightly
different reaction is here explored.
Electrophilic aromatic substitution proceeds by the addition of a positively charged halogen ion to the pi-electron system of an aromatic compound,
forming a halogenated sp3 carbon and a carbocation. The electrons from the carbon-hydrogen bond then return to the pi system, kicking off a hydrogen
ion and restoring aromaticity to the halogenated product. (Figure 1)[1][2]
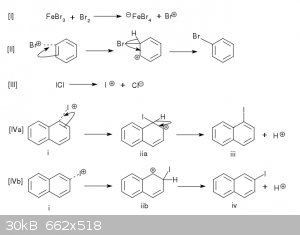
Figure 1. Reactions I and II describe the bromination of benzene described in reference [1] and illustrate the principle of electrophilic aromatic
substitution. Reaction III outlines the formation of electrophilic I+ from iodine monochloride. Reaction IV describes the reaction of I+ with the
naphthalene substrate (i) to form the iodonaphthalene isomers (iii) and (iv).
In [1], iron metal and elemental bromine are reacted to form iron tribromide in situ. The reaction of iron tribromide with additional bromine forms
the electrophilic Br+ cation (Reaction I). Although it is not less dangerous than elemental bromine, iodine monochloride can be produced in small
amounts in a modest lab, with less difficulty than bromine. [3] This substance is a source of the electrophilic I+ cation. (Reaction III)[4]
The above considerations suggest that iodine monochloride could be reacted with naphthalene (i) to produce iodonapthalene isomers (iii, iv).
Potential side reactions include the formation of diiodide compounds; this could be avoided with heavy stirring and an excess of naphthalene. Another
side reaction could occur if the chloride anion from ICl were more likely than the FeBr4- counterion to react with the carbocation intermediates (iia)
and (iib); this could form a chloro-iodo dihalogenated compound. This doesn’t seem to be a problem other examples of ICl-powered iodinations.
[5][6][7]
A more serious concern is the purification of the reaction mixture, in particular the separation of the 1- and 2- isomers expected from the reaction.
Some other reactions (E.g., [5]) seem to show some degree of regiospecificity, but this could be caused by electron-directing substitutents in the
starting material in those cases; thus I would expect roughly equal amounts of the two isomers from this reaction.
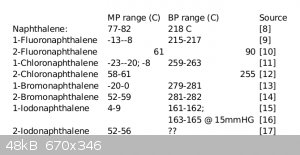
Table 1. Melting and boiling points of naphthalene and monohalogenated derivatives. Note that 1-iodonaphthalene is given similar bp's at
atmospheric and reduced pressure - perhaps ChemSpider is neglecting to mention pressure in its bp reporting?
I couldn’t find a value for the boiling point of 2-iodonaphthalene, but the 1- and 2- isomers of both chloro and bromonaphthalene appear to be quite
similar, suggesting that (vacuum) distillation might be useful to remove the products from the reaction mixture, but not to separate them. I would
also expect them to have similar solubilities. The 2-isomer has a much higher melting point; if the isomers are not soluble in one another, perhaps
one could be crystallized from the other. But I would expect their cosolubility to be high. Perhaps this is a good place for chromatography?
As an additional complication, the 1-isomer is light-sensitive. [16]
Proposed Procedure
1. 1 eq. iodine monochloride is prepared from the elements. [3] This is dissolved in diethyl ether. [4]
Might it be possible and perhaps more efficient to dissolve the iodine in ether, and bubble chlorine through the solution?
2. 10 eq naphthalene are dissolved in ether and placed in a 3-neck round bottom flask. A stream of air is passed in through one neck and taken out
through the other, led into a basic gas washer to remove HCl fumes.
3. The ICl solution is place into an addition funnel, which is fitted into the remaining neck.
4. The apparatus is closed up and the the ICl is added to the naphthalene solution dropwise with stirring.
5. Once all ICl is added and gas evolution has ceased, the apparatus is set up for (vacuum) distillation. Ether and then the products are thus
isolated.
6. [Separation of the isomers??]
7. Profit!
Next Steps
Once the iodonaphthalenes are prepared and isolated, what good are they?
- Aryl halides don’t undergo substitution reactions like alkyl halides

- Aryl halides can be used in the Buchwald–Hartwig amination [18] ... but this requires high-fallutin’ palladium catalysts

- On the other hand, they’re suitable for Grignard reactions [19]
- And maybe the Ullman condensation... glue napthalenes together, or to phenol! [20]
- Or make naphthyl cyanides... [21]
- Or react with PCl3 and sodium to make a naphthyl analog of triphenylphosphine[22]
References
[1] Laboratory Text in Organic Chemistry, 3rd Ed. James Cason and Henry Rapoport.
[2] Organic Chemistry, 6th Ed. T. W. Graham Solomons.
[3] “Iodine chloride - an interhalogen compound” http://woelen.homescience.net/science/chem/exps/Cl+I/index.h...
[4] Da Forezi, L. (2011). Iodine Monochloride. Synlett, (4), 585–586. doi:10.1055/s-0030-1259532
[5] 5-Iodoanthranilic acid. Org. Synth. 1939, 19, 52 DOI: 10.15227/orgsyn.019.0052
[6] 2,6-Diiodo-p-nitroaniline. Org. Synth. 1932, 12, 28 DOI: 10.15227/orgsyn.012.0028
[7] 2-Hydroxy-3,5-diiodobenzoic acid. Org. Synth. 1934, 14, 52 DOI: 10.15227/orgsyn.014.0052
[8] Naphthalene http://www.chemspider.com/Chemical-Structure.906.html
[9] 1-Fluoronaphthalene http://www.chemspider.com/Chemical-Structure.9078.html
[10] 2-Fluoronaphthalene http://www.chemspider.com/Chemical-Structure.60904.html
[11] 1-Chloronaphthalene http://www.chemspider.com/Chemical-Structure.6737.html
[12] 2-Chloronaphthalene http://www.chemspider.com/Chemical-Structure.6789.html
[13] 1-Bromonaphthalene http://www.chemspider.com/Chemical-Structure.6735.html
[14] 2-Bromonaphthalenehttp://www.chemspider.com/Chemical-Structure.10894.html
[15] 1-Iodonaphthalene http://www.chemspider.com/Chemical-Structure.6738.html
[16] 1-Iodonaphthalene http://www.chemicalbook.com/ChemicalProductProperty_EN_CB815...
[17] 2-Iodonaphthalene http://www.chemicalbook.com/ChemicalProductProperty_EN_CB921...
[18] Buchwald-Hartwig Cross Coupling Reaction.http://www.organic-chemistry.org/namedreactions/buchwald-har...
[19] Grignard Reaction. http://www.organic-chemistry.org/namedreactions/grignard-rea...
[20] Ullman Reaction. http://www.organic-chemistry.org/namedreactions/ullmann-reac...
[21] Rosenmund-von Braun Reaction http://www.organic-chemistry.org/namedreactions/rosenmund-vo...
[22] Triphenylphosphine. http://en.wikipedia.org/wiki/Triphenylphosphine
[Edited on 24-12-2014 by mayko]




