
noelpj - 5-12-2004 at 18:29
We have to do an ISU in my chemistry class and this is one of the question which none of me or my friends can get:
Balance the following equation by the half-reaction method.
[Edited on 6-12-2004 by noelpj]
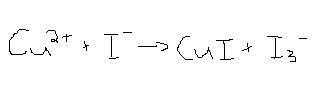
The_Davster - 5-12-2004 at 19:12
2[Cu<sup>2+</sup> + 1e<sup>-</sup> -->Cu<sup>1+</sup>]
+[5I<sup>-</sup> --> I<sub>3</sub><sup>-</sup> + 2I<sup>-</sup>+2e<sup>-</sup>]
_______________
2Cu<sup>2+</sup> + 5I<sup>-</sup> -->2Cu<sup>1+</sup> +
I<sub>3</sub><sup>-</sup>+2I<sup>-</sup>
EDITS: Sub/super scripts
EDIT: next time show some of your failed attempts so that we know you have actually tried the problem.
[Edited on 6-12-2004 by rogue chemist]
[Edited on 6-12-2004 by rogue chemist]
noelpj - 5-12-2004 at 19:39
which half-rxn is the oxidation .. which is the reduction?
The_Davster - 6-12-2004 at 06:04
Answering that would be too much spoonfeeding. All I will say is OIL RIG
bennator - 6-12-2004 at 13:02
LEO GER is sooooo much better than OIL RIG 
alchemie - 12-12-2004 at 14:32
OIL RIG? LEO GER?
Care to explain? Mnemonics or something like that?
The_Davster - 12-12-2004 at 17:05
Yeah OIL RIG and LEO GER are Mnemonics that are taught usually in highschool which are used to remember if a half reaction is an oxidation or
reduction.
EDIT:
O-oxidation
I- Is
L- Loss
R-Reduction
I- Is
G- Gain
L- Loss (of)
E- Electrons (is)
O-Oxidation
G-Gain (of)
E- Electrons (is)
R Reduction
That is why I prefer OIL RIG, there is no assumed words like there is in LEO GER.
[Edited on 13-12-2004 by rogue chemist]



