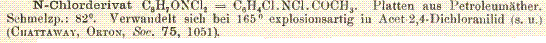And lastly an abstract regarding the conversion of chloramines to bromamines via a bromide salt.
Zawalski, Robert; Kovacic, Peter. "Chemistry of N-halo compounds. 29. A convenient preparation of N,N-dibromoamines" Synthetic Communications (1978),
8(8), 549-62.
Abstract
Ten N,N-dibromoamines, e.g., N,N-dibromocyclohexylamine, Me2CHNBr2, Me3CCH2NBr2, Me3CNBr2, were prepd. in 60-90% yields by treatment of the resp.
N,N-dichloroamine in MeCN or MeOH at 0-5 with a water sol. bromide salt. Several attempts were made to prep. a mixed N,N-dihalo compd., e.g., by
reaction involving an excess of N,N-dichloro-.alpha.-aminoisobutyronitrile with various sources of bromide. |

 , Im not even sure is there are examples of that.
, Im not even sure is there are examples of that.
 )
)