
JAVA - 9-1-2014 at 09:00
Hello guys,
I just get a practical problem: what happens if malononitrile is reacted with sodium in EtOH, is it right that a carbanion intermediate exists?
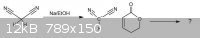
If this is true, what reaction mechanism takes place with this pyranone?
Since I suffering for a while about insertion mechanisms and carbene chemistry it seems impossible for me to explain what the product is?
Reaction scheme is given below
Additional background is very welcome!
UnintentionalChaos - 9-1-2014 at 09:50
There is no carbene there. That is a carbanion. A carbene is a charge-neutral carbon species with two substituents and a pair of electrons (whose
configuration can be singlet or triplet). You have a carbanion (a relatively weak one) and an activated alkene.
http://www.organic-chemistry.org/namedreactions/michael-addi...
solo - 9-1-2014 at 15:32
.....just to clarify, this JAVA is not the java of the hive or any other forum.......java is solo here ....i just thought i clarify that since many
know me as java .....solo/java
JAVA - 28-2-2014 at 08:53
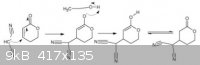
Thank you UnintentionalChaos. It wasn't explained on the university. Then I suppose that this is the mechanism for the 1,4-Michael Addition:
solo: don't mix subjects in scientific discussions, JAVA - solo

