
deltaH - 23-10-2013 at 05:33
I was reading about chemiluminescence and encountered the classic cyalume reagent and mechanism employed in 'glow sticks'. Here's a the figurative
summary of what occurs taken from wiki article on "cyalume".
<img src="http://upload.wikimedia.org/wikipedia/commons/thumb/a/ad/Cyalume-reactions.svg/800px-Cyalume-reactions.svg.png" width="500" />
Seems half understandable, until I get to the second step, the reaction of 1,2-dioxetanedione with a fluorescent dye to yield dye in its excited form.
How the heck does a neutral non-excited molecule like 1,2-dioxetanedione (granted... highly energetic, but so what) couple to a dye molecule
and neatly flip one of its electrons to a high energy state! I mean mechanistically how does this happen?
Let me put it another way, so this dioxetanedione is damn energetic and it would probably VERY easily simply fly apart and form two hot CO2 molecules
that quickly transfer their kinetic energy to the solvent, but how can this bring about an electronic excitation in the dye molecule?!
[Edited on 23-10-2013 by deltaH]
<hr width="80%" />
Just to put things into better perspective, here's the luminol chemiluminescence mechanism also from wiki:
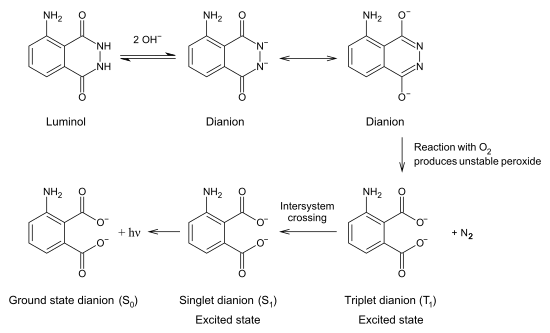
This makes a little more sense because the oxidation of the luminol and subsequent decomposition of N2 generates the phthalate derivative in an
excited triplet state.
So back to the cyalume mechanism, does the peroxide not act on the cyalume like it does on luminol resulting directly in an oxidation to CO2 and
leaving behind a phenolate triplet which goes on to couple with the dye? The dioxetane intermediate proposed sounds like hogwash to me and
mechanistically impossible in the sense that it excites the dye from its ground state?
[Edited on 23-10-2013 by deltaH]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: reduced
image size; merged sequential posts]
[Edited on 23.10.13 by bfesser]
kmno4 - 23-10-2013 at 08:39
If you like wikipedia so much, here you are:
http://en.wikipedia.org/wiki/Peroxyoxalate
Fo me, wikipedia is no source of information.
deltaH - 23-10-2013 at 09:07
Thanks kmno4, pity this article is an orphan, would have made more sense if they had brought this into the other main ones.
Anyhow, yes this makes a little more sense now because they propose that the mechanism proceeds through the formation of excited singlet or triplet
CO2 and that seems possible to me.
But then again, CO2 singlets and triplets are crazy high energy radicals, so I suppose there can be some debate that the peroxylate can form them.
Phenolate radicals seem more plausable because there is at least resonance stabilisation and so this species is considerably less energetic than
singlet or triplet CO2.
For quick reference to those reading this, the mechanism on kmno4's link is:
<img src="https://upload.wikimedia.org/wikipedia/commons/a/a4/Dioxetanedione_decomposition_pathways_revised.gif" />
<del>Stupid link... how can one get the image to work when the file name contains a :D in it's name, or is this not the problem?</del>
[Edited on 23-10-2013 by deltaH]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: del;
disabled smilies; fixed broken image]
[Edited on 23.10.13 by bfesser]
Moderator Request
bfesser - 23-10-2013 at 09:35
deltaH, rather than posting consecutively, please use the <img src="./images/xpblue/edit.gif" /> button to append additional
thoughts to your existing replies. Apparently you haven't noticed that I've been merging many of your double and triple replies into single posts.
Also, I've observed that you rely heavily on the [quote] and [rquote] features—this may be one of the
reasons why you're submitting multiple replies. Please try opening multiple browser tabs and using copy-paste to quote multiple replies in a single
post, when absolutely necessary. Finally, it would be nice if you would simply cut down on your (ab)use of quotes; just address a reply to a username
(as you see me do here).
Of course, this advice applies to equally well <em>all members</em>—I'm not meaning to single you out unfairly
[edit] P.S. "A neutral non-excited molecule" is called a <a href="https://en.wikipedia.org/wiki/Ground_state" target="_blank">ground
state</a> <img src="../scipics/_wiki.png" /> molecule.
The feature you're looking for is "<input type="checkbox" /> Disable Smilies?", directly below the "Message:" text-box input, but the URL you
linked to wasn't the image, regardless. I fixed it for you.
<strong>kmno4</strong>, Wikipedia's alright, but mostly as introductory material and as an easy way to find relevant primary sources
(bottom of every article).
[Edited on 23.10.13 by bfesser]
deltaH - 23-10-2013 at 11:59
Thanks bfesser and noted.

