Cesium Alum Method
Submitted By Robert West and Robert P. Anderson
Checked by Lewis I. Krimen and Therald Moeller
Pollucite is broken into pea sized lumps with a hammer and then ground in a ball mill until fine enough to pass though a 120-mesh sieve. One hundred
grams of screened pollucite is mixed with 400 ml of 50% sulfuric acid (7.1 M) in a 1-L round-bottomed flask and refluxed gently for 30 hours. The
mixture is diluted with 250 ml of water, heated to boiling, and filtered with suction, using a large, coarse, sintered-glass funnel. The silica
residue is washed well with hot water.
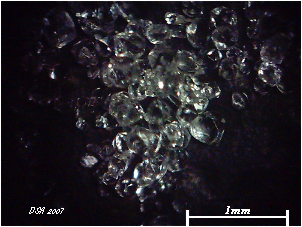
The hot solution is cooled to 0C, and the well-defined octahedra of cesium alum are collected on a sintered-glass funnel and washed with cold water.
A small second crop of crystals is obtained when the filtrate is concentrated to about 450 ml and cooled. The yield is about 110 g., or nearly 90%
(for Varutarask pollucite).
Properties
Cesium alum crystallizes in colorless octahedra which melt at 117 C. This compound is more insoluble then the other alkali-metal alums and is
remarkable for its high temperature coefficient of solubility. At 100C, 12 is soluble in 100 g of water; at 0C, only .19 g is soluble. Because of
this property, the cesium compound is readily prepared in a high state of purity by recyrstalizing it from water. |