Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds which are categorized as phenols (sometimes called phenolics). Depending on the temperature, cresols can be solid or liquid because they have melting points not far from room temperature. Like other types of phenols, they are slowly oxidized by long exposure to air and the impurities often give cresols a yellowish to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell.
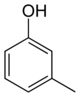
In its chemical structure, a cresol molecule has a methyl group substituted onto the benzene ring of a phenol molecule. There are three forms of cresols that are only slightly different in their chemical structure and physical properties: ortho-cresol (o-cresol), meta-cresol (m-cresol), and para-cresol (p-cresol). These forms occur separately or as a mixture. The word tricresol can be used as a synonym for cresol where it means a mixture of o-, m- and p-cresols.
http://en.wikipedia.org/wiki/Cresol

