
dangerous amateur - 27-4-2012 at 03:18
Another question:
I want to heat an a dilute aqueus solution with several salts in there under reflux, but I'm lacking a condenser.
Now I'm really lacking experience here, but when I heat such an erlenmeyer with screwcap
http://www.vitrum.cz/flask-acc-to-erlenmeyer-with-screw-cap-...
to maybe 80°C - will it blow?
When do you expect it to blow?
Was only some idea that just came to my mind. The long bottleneck would somehow work as a condenser 
edit:
OK, stupid dangerous idea...
Please write your opinion anyway.
I just though about some long piece of glasspipe or something with some wet rags placed on it, left open at the end. Or some other home improvised
ghetto setup...
[Edited on 27-4-2012 by dangerous amateur]
[Edited on 27-4-2012 by dangerous amateur]
Bot0nist - 27-4-2012 at 06:30
Don't reflux in a closed system. That's not the idea, to trap all vapors in the small flask during heating. The idea is to provide a path where the
vapors will be cooled back down to the liquid phase and run back into the reaction before they can escape out the top of the open condenser. Any rise
in pressure from the expanding air/vapor goes out the top of the condenser, but any vapor with a boiling point higher than the temperature of the
coolant running through your condenser jacket will not escape, as it will revert to liquid. Just rig a piece of pipe sealed to the neck and going
straight up from the flask, with it's top unobstructed, and wrap it in wet paper towels. You should never be boiling a liquid with nowhere for the
vapors and pressure to go.
Even placing a RBF filled with ice on top a beaker works for short refluxes and sublimations (It shouldn't be sealed, just sitting on there). When
running reflux with a proper condenser the top of the condenser stays open the whole time. Any water/solvent in the vapor phase is "re-liquefied", AKA
condensed, by the efficient cooling of the condenser by running cold water/coolant through the jacket. This allows a reaction to be boiled for ages
with no loss of solvent, and no dangerously increasing pressure inside a capped reaction/boiling vessel.
Be Safe.
[Edited on 27-4-2012 by Bot0nist]
dangerous amateur - 27-4-2012 at 11:51
Sorry for the stupid proposal.
I'll try to get a cheap little condenser, I prefer proper solutions...
Sure. Sorry for the stupid proposal...
ManBearSwine - 27-4-2012 at 20:17
This is what mine looks like. It works pretty well.
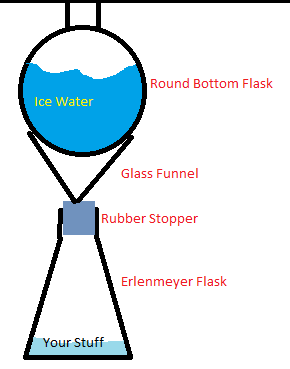
ivo - 31-7-2013 at 05:39
manbearswine your reflux setup looks good but it doesn't have a hole for the pressure to stabilize either, why don't you try putting a round-bottom
flask filled with ice-cold water on top of a beaker with a spout, so that the pressure goes out and everything is safe.
bbartlog - 31-7-2013 at 06:21
The glass funnel and the RBF in his picture aren't going to form an airtight seal, but even if they did, a little overpressure would lift the RBF and
allow gas to escape. I think the main caveat with his setup is that finding a glass funnel that fits a hole in your stopper isn't totally trivial.
sargent1015 - 31-7-2013 at 09:10
Or just add solvent throughout the refluxing process, kinda wasteful, but if it's water still cheap. Only problem then is making sure the water is the
same temp when you add them so you don't have to wait for the reaction to come back up to temp.
bfesser - 31-7-2013 at 09:19
<strong>Bot0nist</strong> is correct; for aqueous reflux, just put an appropriately sized round bottom flask (RBF, or an <a
href="http://en.wikipedia.org/wiki/Evaporating_dish" target="_blank">evaporating dish</a> <img src="../scipics/_wiki.png" /> of H<sub>2</sub>O ice slush on top of a a beaker. There's no need to
complicate things. If you're doing a sublimation, however, put a single tight wrap of <a href="http://www.2spi.com/catalog/supp/parafilm.php"
target="_blank">Parafilm</a> <img src="../scipics/_ext.png" /> around the joint between the RBF and the beaker to keep atmospheric
moisture from condensing and dripping inside the setup, and fill the RBF with slush <em>after</em> sealing the apparatus.
of H<sub>2</sub>O ice slush on top of a a beaker. There's no need to
complicate things. If you're doing a sublimation, however, put a single tight wrap of <a href="http://www.2spi.com/catalog/supp/parafilm.php"
target="_blank">Parafilm</a> <img src="../scipics/_ext.png" /> around the joint between the RBF and the beaker to keep atmospheric
moisture from condensing and dripping inside the setup, and fill the RBF with slush <em>after</em> sealing the apparatus.
<strong>ManBearSwine</strong>, I see many disadvantages to the apparatus in your diagram. First, you're risking snapping the funnel off
at the stem by putting that much weight on the cone. Second, you'll want to use a powder addition funnel, as liquid will 'chug' in the thin stem of a
standard funnel. Fourth, it's annoying to find a stopper that fits both the Erlenmeyer neck and the funnel stem. Fifth, an Erlenmeyer reduces the
headspace available for containing splashing and bumping. Sixth, your apparatus is needlessly tall and would require clamping to avoid toppling over.
And so on…
[Edited on 31.7.13 by bfesser]
sonogashira - 31-7-2013 at 13:32
Maybe you could heat it to a few degrees below boiling point, and save yourself the trouble of condensing anything. What is so great about boiling
point, as compared to a few degrees below boiling point? The energy you add is taken up in the fluid to gas enthalpy of the solvent, rather than
passing on to the reactants, is it not? Maybe it makes people feel better to see it boiling rapidly, like cooking potatoes or something?
With a long enough air condenser, or chemically resistant length of plastic pipe, perhaps, there is no particular need for water cooling at any time.
[Edited on 31-7-2013 by sonogashira]
bfesser - 31-7-2013 at 15:05
Because you have to actively control "a few degrees below boiling point;" so unless you have the equipment, it's easier to just reflux. Besides,
you'll still get significant evaporative loss. An ice filled RBF or a condenser are <a
href="https://en.wikipedia.org/wiki/Standard_operating_procedure" target="_blank">SOP</a> <img src="../scipics/_wiki.png" /> for a
reason—they're reliable, easy, and cheap. The more you complicate things, the more prone you are to failure (see <a
href="http://en.wikipedia.org/wiki/KISS_principle" target="_blank">KISS Principle</a> <img src="../scipics/_wiki.png" /> .
.
sonogashira - 1-8-2013 at 04:49
Good point! A method that I sometimes use is to heat a bottle slowly on a sand bath to near-boiling point, then turn off the heat and stopper the
bottle. This way, no evaporation losses occur, and no additional heat is added to the system - the stopper is sucked in slightly due to the formation
of a slight vacuum within the vessel. The sand keeps the vessel warm for a good amount of time, several hours usually. One can of course reheat it
every few hours to maintain a good rate of reaction, being careful to loosen the stopper as soon as possible, or else it will go pop! and hit the
ceiling. With limited resources, it provides a good alternative to the water- and heat-wasting techniques of usual reflux, especially for doing many
parallel-reactions on a small scale, such as optimizing yield etc.
It's a particularly good method especially for preparing air sensitive salts, if you have no access to inert gasses either. I have made many
ruthenium-triphenylphosphine based catalysts using this method, which would otherwise call for nitrogen and a schlenk line. The slow heating to
boiling point removes dissolved oxygen from the bottle, and stoppering the bottle prevents any oxygen from being reabsorbed by the solvent.
[Edited on 1-8-2013 by sonogashira]



 of H<sub>2</sub>O ice slush on top of a a beaker. There's no need to
complicate things. If you're doing a sublimation, however, put a single tight wrap of <a href="http://www.2spi.com/catalog/supp/parafilm.php"
target="_blank">Parafilm</a> <img src="../scipics/_ext.png" /> around the joint between the RBF and the beaker to keep atmospheric
moisture from condensing and dripping inside the setup, and fill the RBF with slush <em>after</em> sealing the apparatus.
of H<sub>2</sub>O ice slush on top of a a beaker. There's no need to
complicate things. If you're doing a sublimation, however, put a single tight wrap of <a href="http://www.2spi.com/catalog/supp/parafilm.php"
target="_blank">Parafilm</a> <img src="../scipics/_ext.png" /> around the joint between the RBF and the beaker to keep atmospheric
moisture from condensing and dripping inside the setup, and fill the RBF with slush <em>after</em> sealing the apparatus. .
.