
Yttrium2 - 2-11-2022 at 11:56
Vacuum fractional distillation is used to seperate compounds with close boiling points that would otherwise be difficult/impossible to distill with
normal distillation setup.
There is a lot of information about vacuum distillation on the web, but what I am trying to find out is when specifically it can be used?
Take nutmeg for example, it contains several fractions--
Could they be isolated?
Above all, I'm trying to understand when, and when not, vacuum distillation is utilized.
In the picture is that a air bleed tube going into the distillation flask?
[Edited on 11/2/2022 by Yttrium2]
Yttrium2 - 2-11-2022 at 11:58
And is vacuum distillation done by cranking up the heat on the hotplate and then watching for spikes/plateaus on the thermometer, before turning the
cow?
Did someone previously state that the vacuum levels cannot be adjusted?
Would I even need a cow?? Couldn't everything that boils off below the desired temperature simply be discarded?
[Edited on 11/2/2022 by Yttrium2]

Yttrium2 - 2-11-2022 at 12:12
Is it as simple as collecting the right fractions-- the one with the desired boiling point??
Yttrium2 - 2-11-2022 at 12:14
What would be a good vacuum distillation experiment?
Yttrium2 - 2-11-2022 at 12:21
I guess one could not discard the stuff that comes over first without breaking the vacuum.
So the cow is likely essential.
Chemgineer - 2-11-2022 at 13:12
I suppose you could look up the boiling temperature vs pressure graphs for various liquids and if the gap between temperatures increases as the
pressure reduces then vacuum will help you.
j_sum1 - 2-11-2022 at 16:11
Cow is not essential. One method is to break the vacuum between fractions. This likely means you change the temp/pressure conditions between
fractions as well, which might be advantageous.
However, big changes to P/T conditions in the middle or a process are something to generally be avoided. In a normal setup, P is kept constant and
the temperature at the still head informs you as to what is happening in the system. You lose this information if you break the vacuum.
There is much to learn about simple distillation procedures before adding the complexity of a vacuum. Unless there is a particular need, I would be
sticking with atmospheric pressure for most situations.
Yttrium2 - 3-11-2022 at 09:19
When would it be beneficial to add a vigreux?
I think a short path vacuum distillation would be more suitable for seperating the compounds in essential oil, would it?
Also, is ever a reflux column (vigreux) added to short path distillation?
Tsjerk - 3-11-2022 at 09:24
Depends on which compounds in which oil.
Yttrium2 - 3-11-2022 at 09:41
Wouldn't the pear shape flask prevent magnetic stirring?
Both devices are considered short path distillation.
In which instance is the micro short path distillation apparatus used?
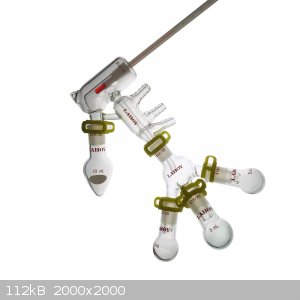
[Edited on 11/3/2022 by Yttrium2]
violet sin - 3-11-2022 at 14:30
Quick look has nutmeg oil "approximately 90% terpene hydrocarbons" as listed there have BP around 150-180°C ... The safrole you want is in the
232-234°C BP area. Wiki didn't have BP for the other components of phenolic character listed.
I'd look closely at what would be trying to come over with it. Price of nutmeg oil with 90%+ losses for small amounts of recreational fun sounds like
an overall failure economically. Personally I'd just not do that.
Your goingt o have to make a list of what's there, what easily leaves, what your left with. Does 170°C damage anything by default. Might have to
use vacuum any way. But more gaming it out needed yo. I'd love to see a sheet of paper littered with effort in your handwriting. Think you'd answer
many of your questions with an organized attack on the subject. Keep a binder, book or note pad and try to work it out.
Perhaps I'm the only one that feels this way? But you would learn more doing the work. Otherwise just be a consumer and enjoy someone else's
reliable time and effort with consumables
-VS
As always , not trying to be an ass or hurt feelings. Best of luck
