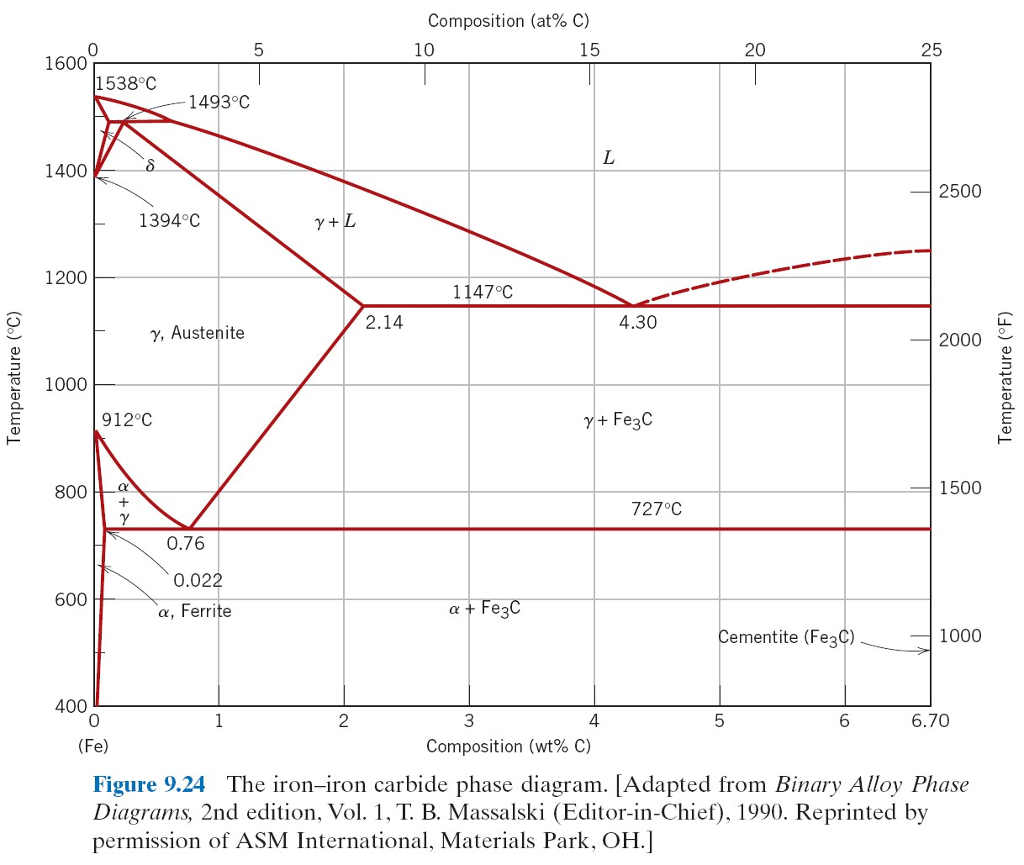
Cast Iron has a carbon content of typically 4.3% by mass. That is the eutectic point of the carbon iron system. Eutectic temperature is 1147C
Properties of cast iron differ significantly from steel.
Steels of various kinds have properties determined in part by the microstructure which is related to the heating/cooling history around the eutectoid
point (0.76%C and 723°C)
At this concentration of carbon the steel melts at around 1500°C. Cooling passes through the austenite phase and then the ferrite phase.
A higher melt temperature is required in practice. Typical slag temperature is around 1630°C, and it needs to be molten to effectively carry away
impurities.
I have not watched the video you posted, but the previous one I did watch is presumably similar. I would estimate that he achieved a temperature just
shy of 1600°C which is just enough to have an effective slag. And my guess is that he ended up with a high carbon steel rather than a cast iron:
maybe 1% carbon.
Of course this is all guessing, but it does give a ball-park answer to your question.
The iron-carbon phase diagram is a complex beast. (Simple version below) But it has features that mean that steel properties are very manipulable.
This is one of the reasons why steel is so ubiquitous.
[edit - post better diagram]
[Edited on 4-9-2022 by j_sum1] |






