
Fery - 5-8-2022 at 10:38
this is the final piece from the trilogy of beta naphthol ethers, I had some old allyl bromide so I used it in this synthesis
referrence:
http://community.wvu.edu/~josbour1/Labs/F2016/Exp%2012%20-%2...
Attachment: Exp 12 - Williamson Ether Synth.pdf (191kB)
This file has been downloaded 386 times
calculations:
M(2-naphthol) = 144,17 g/mol
M(NaOH) = 40,00 g/mol
M(CH2=CH-CH2-Br) = 120,98 g/mol.
0,25 mol 2-naphthol = 36,0 grams
0,25 mol NaOH = 10,0 grams
0,25 mol allylbromide = 30,2 grams (21,6 ml)
Experiment:
36,0 g of 2-naphtol and 175 ml of 99,8% isopropanol were added into 1 L 2-neck RBF equipped with Liebig reflux condenser and dropping funnel.
2-naphthol dissolved quickly (endothermic). 10,0 g NaOH pellets added which dissolved in 1 hour of reflux with rapid magnetic stirring at max RPM and
then the solution was cooled down to approx 50 C. Maybe added crushed NaOH would dissolve faster but that would introduce more unwanted water as it is
very hygroscopic. Ethanol could be probably better solvent but anhydrous ethanol is devilishly expensive in comparison with cheap anhydrous
isopropanol.
30,2 g of allylbromide from dropping funnel was added dropwise into circa 50 C warm reaction mixture while stirring during 15 minutes, then the
mixture was refluxed for 1 hour with magnetic stirring on oil bath (NaBr precipitation).
Liebig condenser rearranged from reflux to distillation and 125 ml of isopropanol distilled out while magnetic stirring on oil bath (a lot of NaBr
precipitate observed). 200 ml of hot water added, stirred, inorganic salts dissolved.
Using separatory funnel the bottom layer aqueous phase was discarded, upper organic layer with the product was kept.
Washed with 200 ml 2% NaOH, bottom organic layer kept, upper aqueous layer discarded.
Washed 2 times with 200 ml of water, bottom organic layer kept, upper aqueous layer discarded.
Hydrodistilled using lazy simple distillation from the same flask which was used for the reaction. First 100 ml of water phase from distillate
discarded, then water from the condensate reused by pipetting it from receiver into dropping funnel and returning to distillation. Density of the
product is only slightly more than the density of water, most of the product was on the bottom of receiver, but a little of it also on the surface
(that's why pouring not good, pipetting from the middle of the height of the condensate from the receiver was the right way to go).
As the product did not solidify and was slightly denser than water, maybe using Dean-Stark trap would make its hydrodistillation more automatic at the
cost of some product returning back on the surface of the condensate from the receiver which does not manage to sink to the bottom of the receiver
quickly enough.
It bumped terribly when using 5 small quartz stones (circa 5 mm size), which was overwhelmed by adding 10 more stones of circa 10 mm size (some
remainders of gravel used to mix concrete from the time of building a house).
A little of the product (from the first 100 ml of distillate to be discarded after pouring off most of water) was put into freezer where water and
product solidified (product did not solidify in fridge at +4 C), then was transferred into fridge to +4 C to melt the ice while product still solid.
Later also the product melted so its melting point could be estimated between 0-5 C (I hope that low m.p. is not due to low purity).
Product pipetted and separated from water using a pipette with rubber bulb for pipette and dried with anhydrous Na2SO4.
Yield 22,4 g of yellow oil (some contamination passed hydrodistillation in microdroplets carried by steam into that few liters of distillate).
I did not find m.p. of the beta compound, I found only alfa here:
https://www.chemicalbook.com/ChemicalProductProperty_EN_CB18...
1-allyloxynaphthalene m.p. -10 C, density 1,048+-0,06 g/cm3
reactants

NaOH pellets on bottom of solution of 2-naphthol (low purity which caused black color) in isopropanol, NaOH dissolved unwillingly only later after 1
hour of reflux and rapid magnetic stirring (perhaps ethanol would be better but anhydrous ethanol is devilishly expensive in comparison with cheap
isopropanol)

allyl bromide (an old commercial product) charged into dropping funnel
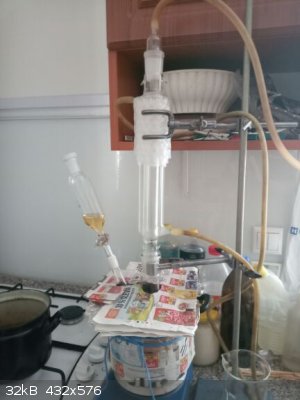
after dropping allyl bromide into reaction the NaBr formation observed very soon

after addition of all allylbromide and at the end of reflux on oil bath more NaBr crystals

most of solvent distilled out (125 ml from formerly 175 ml so circa 50 ml stayed at the end) while magnetic stirring and oil bath (to prevent local
overheating)

after addition of warm water the product in upper layer, water with dissolved NaBr in bottom layer, transferred into separatory funnel


washing with 2% NaOH (note the density of the product circa 1,04 g/cm3, density of such solution of NaOH circa 1,01 g/cm3) - product in bottom layer,
water phase in upper layer (on common light hardly to distinguish as the water phase was discolored, using LED hand light revealed the border of the
layers better)

first washing with water (upper phase), product in bottom layer

second washing with water (upper phase), product in bottom layer

hydrodistillation of the product (lazy simple distillation, discarding first 100 ml of water from distillate, then reusing water from distillate and
returing it back to distillation through separatory funnel)

most of the product at the bottom of receiver




but a little of the product also on surface due to surface tension effect, that's why pouring off the water from distillate not good, better to
pipette the water from the middle of the height of the distillate (using a bulb for pipette - part of it visible in the upper right corner)

collected product after the hydrodistillation was completed

the product was separated from water and collected into an Erlenmeyer flask using the pipette and bulb (pouring wouldn't work !!!, maybe using
separatory funnel would work well, but every pouring causes bottom drops to touch the surface at which it creates oily eyes on the surface)

dried with anhydrous Na2SO4

decanted into a vial (color not good, but such a small amount not worth for vacuum distillation)

I hope the melting point was not so low due to impurities, but here an attempt to estimate it - it did not solidify in fridge at +4 C, but it did in
freezer at -18 C with a little of water with it, then it was transferred into fridge to +4 C where the ice melted and at that moment the product
stayed still solid, liquid water was quickly poured off and the product returned immediately into fridge at +4 C - here solid product immediately
after pouring off just melted ice

later the product started to slowly melt in the fridge at + 4 C (and later become completely liquid and transparent), so I estimate its m.p. somewhere
between 0 (because when ice melted it was still solid) and +5 C (fridge temperature set to +4 C but it oscillates), note this was the product from
first 100 ml of distillate which was discarded, not from the main fraction...

