
ChemistryGhost - 30-6-2021 at 05:51
Hello. I was wondering if tosylation of sesamol and reduction with sodium borohydride in ethanol would yield 1,2-Methylenedioxybenzene. I was also
thinking zinc dust and heat.
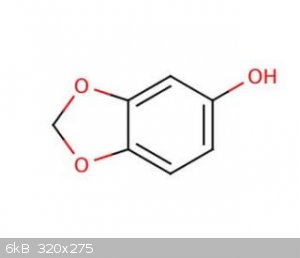
Antigua - 30-6-2021 at 06:10
Why not just go for catechol methylenation...? Proven, good yields.
Catechol can be prepared from salicylaldehyde or a hundred different compounds.
karlos³ - 30-6-2021 at 07:28
Tosylation followed by reduction using NaBH4/NiCl2 would indeed yield benzodioxole.
Mateo_swe - 20-7-2021 at 22:49
I would save the Sesamol for something better/more interesting.
Methylation of catechol with DCM works good and catechol is easily purchased.
Link below is a thread about this procedure and in that thread are other links of interest regarding this subject.
http://www.sciencemadness.org/talk/viewthread.php?tid=76069#...
Syn the Sizer - 21-7-2021 at 06:58
Though the phenolic reduction works, I have to agree from catechol would be a better route. Simply because catechol, sodium, and DCM are so cheap as
compared to NaBH4.
Edit:
I.intend to do the synth using the catechol route soon. I have some DCM but not quite enough so I want to get more first.
[Edited on 21-7-2021 by Syn the Sizer]
Syn the Sizer - 21-7-2021 at 07:06
I have said it before and will say it again. I like smelling thing, never huff, but I waft everything. I want to know what stuff smells like for
various reasons. That is actually 1 reason I want some benzenedioxol lol. I know, sounds stupid.
