
Triflic Acid - 5-6-2021 at 08:22
I'm trying to make dimethyl carbonate from ethylene carbonate by transesterification. Does anyone have a good prep for this?
njl - 5-6-2021 at 10:17
As you know ethylene glycol is less volatile than methanol so beyond using a large excess of methanol and praying to the equilibrium gods you won't
have much luck. This is an appropriate amount of detail to go into based on your question.
As a fairly active member I have seen your posts and threads. Since my words are basically gospel round these parts I'll say this based on my own
experience: try to stay focused. I see you post about an idea, flesh out and defend that idea, realize that despite your defending the idea it needs
work (don't worry - this is how it's supposed to happen), rework your idea, rinse and repeat with new thread and new question. This creates clutter
and breaks the stream of consciousness between us.
You could make a new call and response thread for each of your will this work questions, but I'd argue that a more valuable discussion comes
from will X work --> yes/no and why --> reformulate X to X.1 based on new understanding --> will X.1 work all together in one place.
To be clear there is no ill intent in this reply, nor am I specifically speaking to this idea. I spent a long time writing this out because it's
important to me (and the spirit of the forum) that we share knowledge, and we can only do so if we share mindfully.
Opylation - 5-6-2021 at 13:43
If your goal is to truly prepare dimethyl carbonate I have no tips or tricks to give. But I do want to say, since the massive push to phase out DCM in
paint thinners and strippers one of the “Green” chemicals that has replaced it is dimethyl carbonate. You may want to do some deep dives into
paint strippers sds’
[Edited on 5-6-2021 by Opylation]
Triflic Acid - 6-6-2021 at 09:08
njl, I'll try to follow that better in the future. Opylation, I don't know of anywhere I can get the paint stripper, but I did end up finding a seller
for pure dimethyl carbonate. I'll buy it from him, since it is the cheapest as of now.
rockyit98 - 6-6-2021 at 12:51
what is your objective?
is it a demonstration, a practical, for a paper or you just need it as a solvent?
why not an autoclave?
you only need autoclaves for reactions that use or end up making solids. if all ingredients and products are gas or liquids small CO2 can can be used
in some cases.
urea react with high boiling point alcohols (I.E like ethylene glycol) under low pressures in presence of some catalytic compounds.
Hope I helped. feel free to ask if needed.
clearly_not_atara - 6-6-2021 at 14:02
I think potassium methoxide as prepared from K2CO3 and MeOH will react with ethylene carbonate to give methyl hydroxyethyl carbonate, MeOC(=O)OEtOH,
because its conjugate base is somewhat weaker. This can then be oxidized with chromic acid to methylcarbonatoacetic acid, which in turn may allow you
to dry distill Me2CO3 from its alkali salts.
But it's also plausible to me that methyl hydroxyethyl carbonate is actually what you want and you don't realize it. As far as I'm aware,
unsymmetrical carbonate diesters prefer to transfer the methyl over the other groups.
Another possibility I thought of is to prepare potassium methyl carbonate by gassing a KOMe solution with (excess) dry CO2, then add sodium (or
potassium!) methyl sulfate and heat. Hopefully you get this:
KMeCO3 + NaMeSO4 >> Me2CO3 + NaKSO4
That avoids chromic acid — or ethylene carbonate, for that matter!
EDIT: It would be smarter to prepare the much better ethyl methyl carbonate by using sodium ethyl sulfate in the second method. MEC has a lower
freezing point, a higher boiling point, and still primarily methylates rather than ethylates.
MEC solvent properties: https://www.lestudium-ias.com/system/files/phadke_et_al-2018...
MEC as a methylating agent: https://www.researchgate.net/publication/232238560_Alkyl_Met...
[Edited on 6-6-2021 by clearly_not_atara]
rockyit98 - 15-6-2021 at 10:35
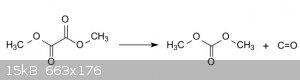
Dimethyl oxalate Decarbonylation gives dimethyl carbonate
Dimethyl oxalate can be obtained by esterification of oxalic acid with methanol using sulfuric acid as a catalyst.
CO is toxic!
more digging up papers are needed.
Triflic Acid - 15-6-2021 at 10:39
That might be interesting to consider.
Chemi Pharma - 18-6-2021 at 22:34
From dimethyl oxalate and sodium methoxide. Patent attached.
ChemPlayer has a video at BitChute on how to prepare dimethyl oxalate from esterification reaction between oxalic acid and methanol catalised by
sulfuric acid.
Attachment: Dimethyl carbonate from dimethyl oxalate and sodium methoxide.pdf (269kB)
This file has been downloaded 386 times
rockyit98 - 19-6-2021 at 11:24
https://prepchem.com/synthesis-of-dimethyl-oxalate/
Keras - 20-6-2021 at 00:30
What are going to use the dimethyl carbonate for? As a methylating agent? It is green, but rather inefficient, if memory serves.
Triflic Acid - 17-7-2021 at 13:29
Solvent
Mateo_swe - 20-7-2021 at 09:25
I bought some DMC from a chemical supplyer, but it was 50Euros for a liter.
If you are inside EU you can find it for sale and shipped to your door, somewhat expensive though.
Maybe there are cheaper places to get it, beeing a "green" alternative i guess it should become more frequently used.
Sometimes it listed as Carbonic acid dimethyl ester but its DMC (methyl carbonate, dimethyl carbonate).
