
JohnnyBuckminster - 8-5-2021 at 09:51
Perfluorpolyeter is used in vapour phase reflow soldering because of being inert up to high temperatures. It is sold by, for example, Imides,
who described it as
CF3 – O – CF – CF2 – O – CF – CF2 – O – CF – CF2 – O – CF – CF2 – ……………… – O – CF3 CF3 CF3 CF3 CF3
"The liquid polymers of the Galden FM series are built according to this principle."
Does this structure make any sense? Can you have "– CF2 – O – CF – CF2 – ".
If anyone can make a drawing in for example ACD/ChemSketch and share the file that would be great.
If anyone is interested in how the substance is used in the DIY manufacturing of PCBs, watch
https://youtu.be/D28uSzCs7-k?t=620
Texium - 8-5-2021 at 12:06
I would expect that all the CF3s listed at the end there are supposed to be attached to the CFs, since -CF- is missing one substituent.
JohnnyBuckminster - 8-5-2021 at 19:07
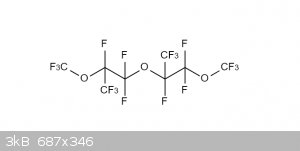
Ok, that make sense. The extra CF3's at the end should be attached to the -CF- groups, like in the image below.
Texium - 8-5-2021 at 19:16
Exactly!
Dr.Bob - 10-5-2021 at 10:30
That makes sense, likely made from hexafluoropropene (a cheap byproduct of Teflon production), which would be epoxidized and then polymerized. Short
chains of this are used as solvents for PTFE production and considered long lasting enviromental issues.
JohnnyBuckminster - 11-5-2021 at 11:39
In the Hydrogen–deuterium exchange process covalently attached hydrogens are replaced with deuterium over time, I read this in Wikipedia.
Would a similar approach work to manufacture hexafluoropropylene, i.e exposing propylene to an excess of fluoride ions over time? Would the hydrogen
spontaneously be replaced with fluoride, like in the scheme below?

DraconicAcid - 11-5-2021 at 11:50
I very seriously doubt it.
Dr.Bob - 11-5-2021 at 13:01
There are electrochemical ways to swap F for H, but they are not trivial, mostly done on industrial scale.
Fluoride ions are not likely to displace hydride from a hydrocarbon.
But you can make perfluoropropane by fluoronating perfluoropropylene, using CoF3 as the fluorinating material. Exposing propylene to straight
fluorination would likely create a large explosion.
