wg48temp9 - 28-1-2021 at 09:45
Water tracing dye available from TOOLSTATION. Probably the sodium salt.
Housane - 8-2-2021 at 04:58
Nice find, thanks
unionised - 8-2-2021 at 05:12
Possibly 1% dye mixed with salt.
For £6.3 I might add some the next time I'm buying DIY stuff, and ash it to find out.
Here's the datasheet
https://cdn.aws.toolstation.com/items/coshh/19855.pdf
They also sell xylene which isn't common on the UK OTC market.
[Edited on 8-2-21 by unionised]
wg48temp9 - 9-2-2021 at 03:15
I checked the safety note before I purchased it, that suggests its not cut with anything.
After purchase and before my first post I examined the powder by shaking a small quantity on a white dish to separate any different powders I could
not detect any differences.
I have now attempted to recrystallize some of the powder from water, on a slow cooker hot plate (2 hours about 100C). After all the water
evaporated I was left with about 1mm crystals in a brown amorphous dark brown gunge. I was not able to determine if the crystals were transparent
due to the gunge.
Curiously the original powder or the gunge is not fluorescent including the acid version of fluorescein powder or any of the fluorescent dye powders
I have. Apparently at high concentration and as a solid this is caused by self quenching ie resonance transfer of the excited state to nearby dye
molecules.
But why don't the adjacent dye molecule produce fluorescence ?
wg48temp9 - 10-2-2021 at 09:38
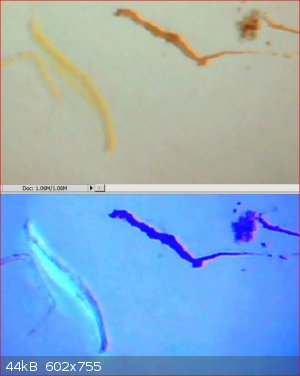
Below are some images of the self quenching effect of the fluorescence at high concentration of fluorescein.
The first image is some smears of fluorescein on white paper taken in room lighting. The brown smears are high concentrations and the yellow smears
are low concentrations (small amount smeared out with a wet finger)
The second image was taken under UV light. Only the low concentration smears are fluorescing.