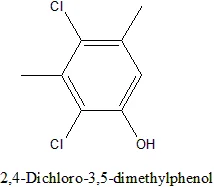
If you are thinking about nucleophile substitution then the answer is no. No because it'd require strong electron withdrawing groups to "activate" the chlorines towards nucleophilic susbstitution. o-and p- substituents relative to the chlorine are required for such an effect, m-substituents have no effect.
Moreover those methyl-groups may flank the chlorines nicely, further decreasing their reactivity.
You read my theory.
Now let's allow others to chime in!