
chemoleo - 25-1-2011 at 18:49
Can anyone recommend one?
Preferably it is unreactive, i.e. tolerated by acylations.
Solvents I'm thinking of are DMF, DCM, NMP, including non-nucleophilic bases.
Silicone polymers seem the material of choice, but there are so many, perhaps someone has some experience and/or advice?
For instance, what do you think of this stuff
http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en...
Sadly they don't give a structure - it'd help!
Magpie - 26-1-2011 at 00:15
Where I worked in industry Dow defoamers were used successfully on paper pulp washers and in a brine evaporator.
This MSDS may help in determining the main ingredient:
http://www1.dowcorning.com/DataFiles/090007b281460737.pdf
chemoleo - 26-1-2011 at 14:41
THanks- but I'm more after an antifoamer that is already anhydrous, and explicitly useful for organic solvents.
The scenario is, a reaction vessel receives constant N2 bubbling, but at times it foams so much that reactants come out at the top end.
Easy, you'll say, just reduce the N2 flow- but that isn't easily done - plus the N2 is required to for mixing. So I thought an antifoaming agent might
be the smartest solution...
vulture - 26-1-2011 at 14:59
Mechanical - if your reaction setup involves ground glass joints, employ a foam breaker:
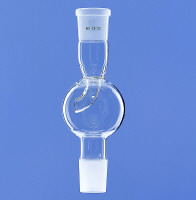
You can also reduce foaming by lowering surface tension of the liquid. I sometimes add dichloromethane, hexane or diethylether for rotary evaporation
of foamy solvents. But with N2 bubbling they'll be gone quite quickly.
Magpie - 26-1-2011 at 15:38
Chemoleo, I wasn't so much thinking that the Dow product per se would be what you're looking for but the silicone name might be of use, eg,
polydimethylsiloxane, etc.
Here's one for the oil & gas industry that is specifically all organic:
http://www.dowcorning.com/applications/search/default.aspx?R...

