
Meister - 8-5-2019 at 07:43
Hello everyone!
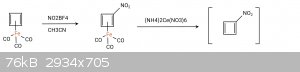
According to wikipedia, cyclobutadieneiron tricarbonyl is capable of electrophilic substitution so in theory, aromatic nitration should work on it.
I present the following synthetic route to nitrocyclobutadiene, which according to my knowledge, should work.
The idea is to nitrate the complexed cyclobutadiene in non-oxidative conditions to prevent the decomposition of the complex and the escape of
cyclobutadiene. The next step is oxidative decomplexation of said complex with ceric ammonium nitrate or a smilar oxidizer to afford
nitrocyclobutadiene in situ, which would be trapped with a diene. I assume the free nitrocyclobutadiene to be more elusive than it's parent compound
since it is even more electron deficient.
Nitrocyclobutadiene should react with dienes, which in turn could lead to new interesting strained energetic structures.
Since I have limited knowledge in chemistry I require help. This is the reason why I came here. Looking at previous threads it seems that this is a
good place to discuss ideas like these. Sorry for the shit-tier picture, I am on mobile. Thanks for any help in advance!
[Edited on 2019/5/8 by Meister]
