
andre178 - 13-4-2010 at 14:58
for example in the picture below, 15% and 83%
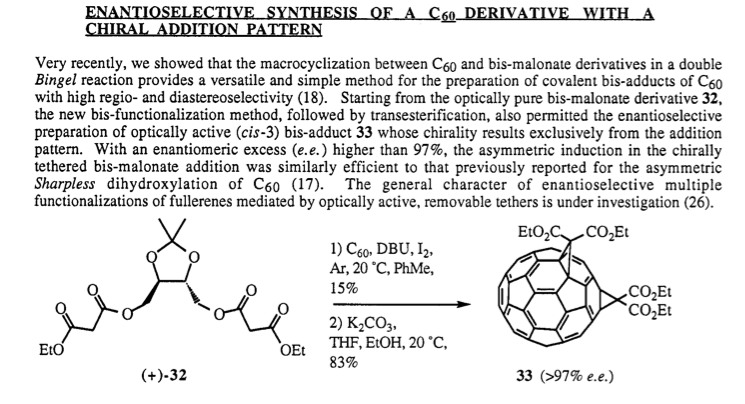
JohnWW - 13-4-2010 at 17:25
It means that the yield from the reaction of the desired product has been that percentage of what would ideally have been obtained if all the least
abundant reagent present had been quantitatively converted to the desired product.
Ozone - 13-4-2010 at 17:26
I could have sworn that I said "yield?" in a previous post? There was no picture then.
O3
andre178 - 14-4-2010 at 08:56
you are right ozone,
It just didn't click, usually yields are on products, of course it makes sense now.
Thanks for the replies
