
smaerd - 23-1-2010 at 16:50
(Hopefully this is in the right section of the board)
So I am a total noob to chromatography but I am curious about how it all works.
So silica is polar?
How does someone consider the proper solvent's to mix for a binary solvent/eluent?
Say the XlogP3 of one of the chemicals in the compound that someone wanted separate was 1.5, and the other was 2.5 .
It was recommended to use EtOAc and MeOH(ratio is unknown). EtOAc seems to be fairly hard to obtain, so I was hoping to switch it out with a solvent
that is similar and just adjust the Methanol to 'fix' the change. Any idea what a good way to approach this would be?
So when using a polar oriented binary solvent and trying to separate polar compounds(redundant but trying to be clear with my limited knowledge), the
most polar of the compounds will elute last?
Any tips, tricks, information, or things I should know would be so, so amazing  .
.
Xenoid - 23-1-2010 at 18:36
Well, I don't know anything much about chromatography, but after a few minutes on Google I think I know more than you!
I feel you should study some of the lecture courses on chromatography that are freely available. For example here:
http://orgchem.colorado.edu/hndbksupport/chrom.html
Why are you so fixated on ethyl acetate when other solvents/solvent mixes can be used, for example hexane (camping stove fuel) and acetone or alcohol
all available cheaply at a hardware store. The tables below should give you some idea. I gather solvent choice is "suck it and see" or "hit and miss",
see what works best.
Edit: Your methanol/ethyl acetate mix could possibly be replaced with methanol/acetone or hexane/acetone in different proportions of course. When you
are in the hardware store, check out the contents of the various "thinners" and "solvents" they are usually mixes of aliphatic or aromatic
hydrocarbons and various ketones, these can be used "as is" or mixed with "pure" solvents to achieve the right results.
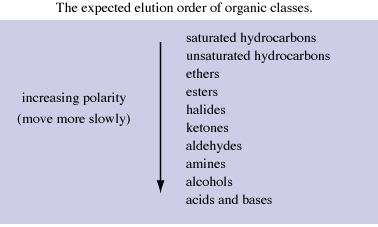
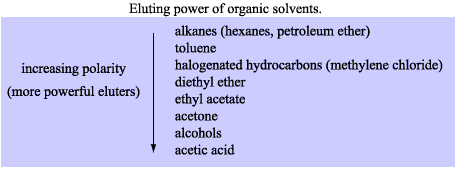
[Edited on 24-1-2010 by Xenoid]
smaerd - 23-1-2010 at 22:08
Thanks I have much to learn. I'm a fairly new chemistry major and just wanna get ahead of the class with experience in the actual handy work.
Time to get back to the books .
.
edit - thinking about using acetone and methanol instead.
[Edited on 24-1-2010 by smaerd]
ziqquratu - 24-1-2010 at 13:57
You want to be careful with methanol when running columns. Too much of it in your mobile phase can dissolve some of the silica, dragging it through to
contaminate your product. The rule of thumb we follow is no more than 10% MeOH.
Anecdotally, acetone can do the same thing, but I've never had the problem (and have run a few columns in 100% acetone).
BromicAcid - 24-1-2010 at 17:28
In my experience, when working to develop a column chromatography procedure (which tend to be avoided at all costs) I use TLC plates to get my
baseline mixture since they give a good idea of the separation of the components, they're quick to run, and they waste a minimum of good material.
Once I get good seaparation on my plates I scale to a column in a buret and finally to a nice full sized column.
smaerd - 25-1-2010 at 16:17
Awesome advice bromic  .
.
Sillica is expensive! TLC plates are pricey in bulk but not too bad singularly. Was looking into diametous earth(spelling is wrong) but for the first
attempt I want standard equipment. Unless someone can help me understand the differences that may be involved swapping silica for another medium.
I'm just trying to figure it all out still. Lot's of research to do still but it's slowly sticking.
The benefit of the separation I will be doing is a lot of the work is cut out for me(others have done it and reported it). It's just a matter of
adjusting the solvents because the etoh ac is just too expensive and unnecessary.
Just trying to figure out how exactly a binary solvent mix can be calculated or approximated to reduce the amount of trial and error involved. I want
to replace ethyl acetate with acetone seems like the most sensible common solvent to do that wit.
Seems like a lot of people avoid chromatography, though this is the part of chemistry that fascinates me(for now)! Couldn't hurt to get a head of the
class either. While their all packing columns with confused looks I'll already have the analyte loaded  .
.
[Edited on 26-1-2010 by smaerd]
[Edited on 26-1-2010 by smaerd]
BromicAcid - 25-1-2010 at 17:40
I believe there might be a thread floating around discussing alternative packing materials for columns such as confectioners sugar and calcium
carbonate. Some of the founding work on chromatography was done in such columns. Although they have some traits that are lacking they are at the
very least cheap.
Cloner - 28-1-2010 at 15:41
The silica used for chromatography is fine but not too fine, and very porous. Too fine and you will need huge pressure. Not fine or porous enough, and
there is hardly any effect.
Powdered sugar was substituted for silica not too long ago in the removal of reaction goo from product on a rather apolar solvent mixture. I found out
it didn't do anything at all, while silica for chromatography gave good results (well, it better be, being expensive). Everything else was the same,
so the silica really is powerful.
Instead of using TLC's to test chromatography, I tend to use pasteur pipettes with a wad of tissue paper in the bottom and some of the column
material. This is much easier than making or buying TLC plates (but if you have them, they are great).
smaerd - 28-1-2010 at 16:21
Ah yes I was reading about the pipette column technique just the other day. Seems like a fairly novel and cheap route.
I take it silica can't be reused even after the solvents have been evaporated? I didn't realize it was feasible to prepare TLC plates, kind of thought
it was manufactured or no dice. This could be something worthy of looking into.
Thanks for the advice lots of things to look into!
entropy51 - 28-1-2010 at 16:57
You might want to look at the chapter on chromatography in the book of projects for the amateur scientist in the forum library. It's quite basic, but
might give you some ideas.
A nice, fine CaCO3 can be made by mixing CaCl2 (ice melt) with Na2CO3 (washing soda) in solution, washing and filtering off and drying the
precipitate.
[Edited on 29-1-2010 by entropy51]
ziqquratu - 28-1-2010 at 18:18
Pipette columns can be misleading - they work great if you have a very small amount of material that is easily resolved, but anything else and they're
near useless. Plus, how are you able to visualise your fractions without TLC anyway? Evaporating each and checking it individually would be a right
pain, particularly once you move up to a large column on a compound with low Rf. And even if you do this, it would be difficult to be certain that
each fraction was in fact pure without some sort of analysis before you mix them together and "unpurify" them!
Seriously, if you can buy the silica, get some TLC plates while you're at it - your life will be SO much easier. And they're not THAT expensive if you
cut them small - I get on the order of 50 plates from a single 20x20 cm sheet (which costs about $6 each in a pack of 25, probably a bit more if
bought individually). The trick is to make good spotters and use them properly so you get nice, tight, small spots and can thus fit many more spots
per plate (I can easily get 6-8 spots on a 1.5 cm wide plate if my spotter is any good).
To make a good spotter, take a Pasteur pipette, hold one end in each hand and heat it in a Bunsen burner (or similar) flame, rotating it constantly.
Once it softens, just before it begins to sag, pull your hands apart in a quick, fluid motion while raising the pipette from the flame. If you do it
right, you should get a nice long, thin capillary that you can chop into several pieces. It can take a few attempts to get it right. Use each one
until it breaks or becomes clogged - just rinse it with some acetone or MeOH in between each use (dip it into the solvent and then blot it onto some
paper towel or similar).
As far as reusing silica from columns - it can be done. If you wash the silica with 10% MeOH in EtOAc (or similar - again, acetone would probably
work), that'll remove most of the residues and you can then reuse the column without repacking a couple of times at least (depending on how well the
wash works - if there's a lot of junk in your crude material that sticks near the baseline, then you will get fewer uses than if you can flush
everything off).
Be careful doing this, though - if you rapidly increase the polarity of the mobile phase, the exotherm that occurs can cause the column to "crack"
(ie. the silica will break into two or more pieces), which will destroy your resolution if you try to reuse it. The best bet is to step up the
polarity slowly: say you're using 50:50 hexanes/EtOAc, increase in steps of ~5% EtOAc until you reach 100%, then increase in two steps of 5% MeOH.
smaerd - 28-1-2010 at 19:06
entropy:
Can't you also make CaCO3 as a by-product precitipitate for sodium hydroxide preperation from, Ca(OH)2 and Na2CO3? Never a bad idea to have some extra
base hanging around either(who knows when the drain will clog)!
edit: Got the book, thanks for the recommendation 
zaqquratu:
Wow thanks so much
I would have never thought of that pipet thing. Yea it does seem as if tlc plates are a necessity while working with a column, I can't see much of any
other way around it unless the compounds were other colors and this was known prior. Edit - or if you had a theile tube and checked the mp and
bps(maybe). Yea nothing is going to be dirty running through the column, a composition of maybe two items(possibly three but that will elute before
the other two) in solution. Good to know about the exothermic reaction!
This forum is such a big help, I'm finally really starting to understand the whole process. If any passer-bys are reading this check youtube for some
good videos on how it works.
Oh the wild world of chemistry 
[Edited on 29-1-2010 by smaerd]
[Edited on 29-1-2010 by smaerd]

 .
.
 .
. .
. .
.
