

 <sub>2</sub>.H<sub>2</sub>SO<sub>4</sub>
<sub>2</sub>.H<sub>2</sub>SO<sub>4</sub>
 Anyways, I had the first editon at another local library and ordered it, but here
is what caught my eye, over one hundred pages on the preparation of hydrazine using numerous methods (Im guessing some interesting curiosity methods)
regardless, amazon has it, but it's $393.12, I mean, come on! I'm not made of money!
Anyways, I had the first editon at another local library and ordered it, but here
is what caught my eye, over one hundred pages on the preparation of hydrazine using numerous methods (Im guessing some interesting curiosity methods)
regardless, amazon has it, but it's $393.12, I mean, come on! I'm not made of money!
| Quote: |
| Quote: |
| Quote: |
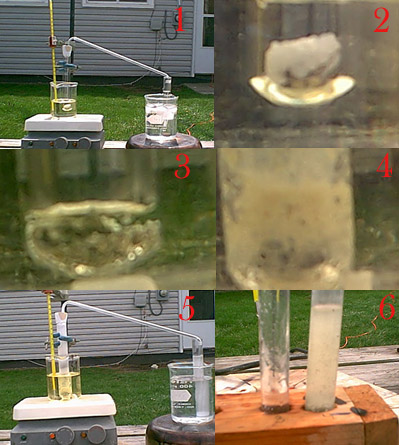

| Quote: |


| Quote: |
| Quote: |
 ).
The reducing agent could be sodium sulfide or something.
).
The reducing agent could be sodium sulfide or something. . I read that whole thread again and still
find no mention of urea DInitrate. Just urea nitrate, nitrourea and dinitrourea.
. I read that whole thread again and still
find no mention of urea DInitrate. Just urea nitrate, nitrourea and dinitrourea. . Also, adding dilute h2so4 would form the sulphate without hazardous explosion hazards, yes? I will report fairly soon :p. Thanks.
. Also, adding dilute h2so4 would form the sulphate without hazardous explosion hazards, yes? I will report fairly soon :p. Thanks.| Quote: |

| Quote: |
| Quote: |
| Quote: |
| Quote: |
| Quote: |
| Quote: |


 , and even
, and even  Been there ,
done that .
Been there ,
done that .| Quote: |
| Quote: |


| Quote: |
| Quote: |
| Quote: |

| Quote: |
| Quote: |
| Quote: |
| Quote: |
 although you will have to watch the speed to keep it from uncoupling in
although you will have to watch the speed to keep it from uncoupling in As well I have felt weird today, not a way I have felt before. I wore a respirator the
entire synthesis, except during filtration.
As well I have felt weird today, not a way I have felt before. I wore a respirator the
entire synthesis, except during filtration.
| Quote: |
| Quote: |
 Transesterfication purified
moonshine anybody ? Pass me the jug
Transesterfication purified
moonshine anybody ? Pass me the jug 


| Quote: |


