
symboom - 10-12-2018 at 18:01
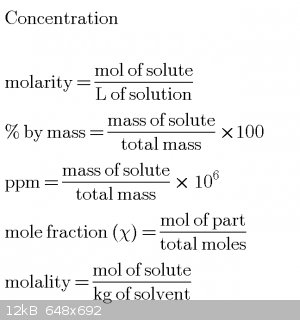
These equations have helped me greatly but it is a problem to find many of these equations many text books have it scattered around the pages. So here
is a quick review all in one place
Equation editor app used
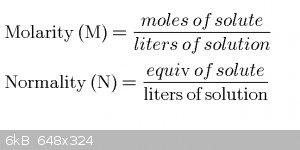
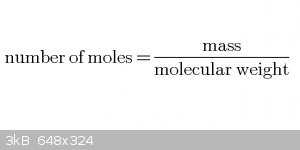
Starting equations

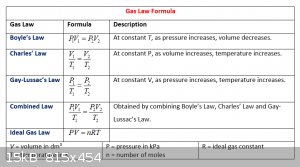
Gas laws

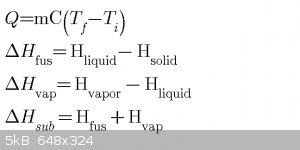
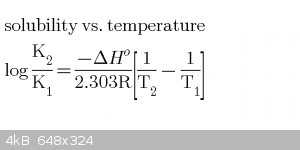
Thermochemistry

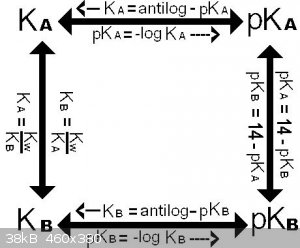
Acid/base
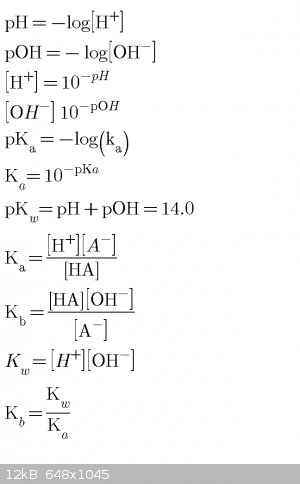
Rate of a reaction


[Edited on 11-12-2018 by symboom]
[Edited on 11-12-2018 by symboom]
CharlieA - 11-12-2018 at 16:55
If you have all those equations down, you have a fine start to understanding some chemistry!
Ubya - 12-12-2018 at 00:51
nerst equation is also useful for redox reactions, and maybe henderson-hasselbalch to calculate buffer solutions
symboom - 12-12-2018 at 06:06
Here is both
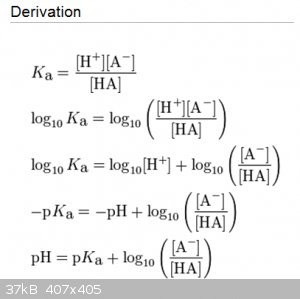
Henderson-Hasselbalch
nerst equation

[Edited on 12-12-2018 by symboom]
symboom - 16-1-2019 at 19:40
Here is a couple more equations
Hope they help

Osmotic pressure...
M is molarity: good old moles per liter.
[Edited on 17-1-2019 by symboom]

