
Filemon - 18-4-2008 at 10:16
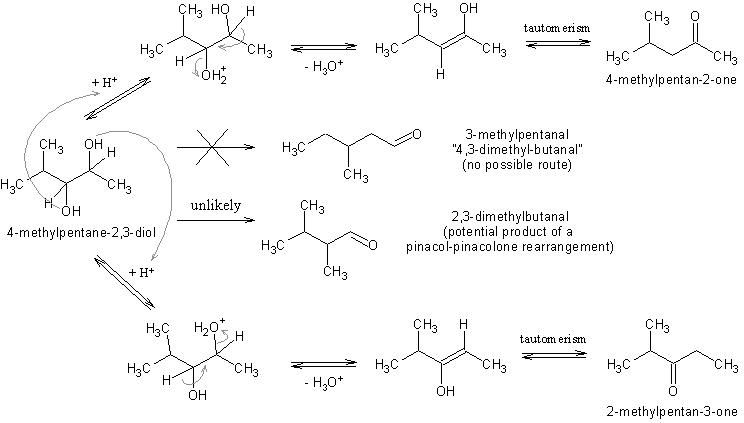
How pinacol rearragement works in this case: 4-methyl-2,3-pentandiol? => 4,3-dimethyl-butanal or 4-methyl-2-pentanone
Sauron - 18-4-2008 at 11:19
One of your proposed products would be a pinacol-pinacolone rearrangement involving a 1,2-methyl shift.
The oter one, the ketone, is not a pinacolone rearrangement at all. No methyl migration. A hydroxyl is protonated, H2) eliminated, an enol forms and
you've got a ketone. No rearrangement.
Nicodem - 19-4-2008 at 02:01
I don't think you can get a pinacol-pinacolone rearrangement on such a glycol. Generally only tetrasubstituted glycols give rearrangement products
unless one of the substituents is particularly susceptible toward 1,2-shifts.
As Sauron told you, the 4-methylpentan-2-one is the product of water elimination. Glycols, their esters and epoxides are prone to give ketones upon
treatment with acids, especially if the cleavage of one of the C-O bonds results in a stabilized carbocation. The other product of such a reaction on
4-methyl-2,3-pentandiol would be 2-methylpentan-3-one. (I'm quite sure this type of reaction was already discussed on this forum so use the search
engine)
4,3-Dimethyl-butanal is an incorrect name for 3-methylpentanal, which however, is not a product of either a rearrangement or elimination.
Bellow is a scheme for the reactions discussed. The mechanisms of H2O eliminations are not really correct since they are greatly simplified. The water
elimination from the glycol is depicted as E2 even though it probably involves a carbocation from which: (a) either a beta-proton could eliminate
giving the enol form of the end ketones; (b) a 1,2-hydride shift could occur resulting directly to the end ketones. But for pedagogical ends such a
simplification is more than enough.
Needless to say, if this is shown on paper it does not mean the reaction actually work and that these ketones can actually be prepared from
4-methyl-2,3-pentandiol (for example, the elimination might require conditions so harsh that the end products become tar).
Sauron - 19-4-2008 at 02:27
My one great publication in JOC was about acetophenone pinacol, or more specifically, its partially stereoselective preparation by using McMurray's
Reagent (TiCl4/LAH) in THF to reduce acetophenone, instead of the usual aluminum amalgam in anhydrous ethanol.
Acetophenone pinacol cannot however undergo the famous rearrangement. So I guess this little trip down memory lane is a wee bit OT.
[Edited on 19-4-2008 by Sauron]
