





Quote: Originally posted by Mailinmypocket  |

Quote: Originally posted by Endimion17  |

Quote: Originally posted by Squall181  |



 .
.Quote: Originally posted by watson.fawkes  |
 The process is called tempering , by the way.
The process is called tempering , by the way.Quote: Originally posted by Eddygp  |
Quote: Originally posted by plante1999  |
Quote: Originally posted by Adas  |
Quote: Originally posted by Number 9  |
Quote: Originally posted by Number 9  |
Quote: Originally posted by kristofvagyok  |

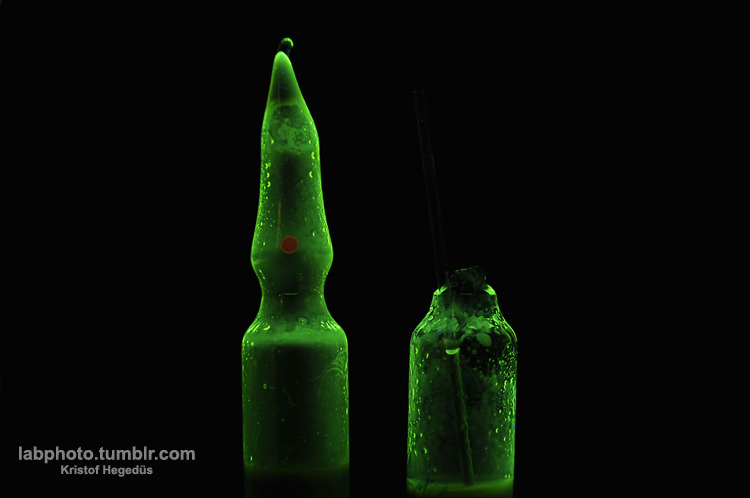
Quote: Originally posted by hyfalcon  |
Quote: Originally posted by Boron Trioxide  |
 ? Isn't this just a pitted piece of SS? If not - how you made that?
? Isn't this just a pitted piece of SS? If not - how you made that?  And I thought we were friends.
And I thought we were friends. 

Quote: Originally posted by Mailinmypocket  |

Quote: Originally posted by Mailinmypocket  |



Quote: Originally posted by Mailinmypocket  |

Quote: Originally posted by Lambda-Eyde  |
 The true high-functioning alcoholics are archaeologists and <a href="https://www.youtube.com/watch?v=q29578ZF_7o"
target="_blank">geologists</a> <img src="../scipics/_yt.png" /> (there's a long-running dispute over who's more <a
href="http://uncyclopedia.wikia.com/wiki/Geologist#Geologists_and_Alcohol" target="_blank">alcoholic</a> <img src="../scipics/_ext.png"
/>
The true high-functioning alcoholics are archaeologists and <a href="https://www.youtube.com/watch?v=q29578ZF_7o"
target="_blank">geologists</a> <img src="../scipics/_yt.png" /> (there's a long-running dispute over who's more <a
href="http://uncyclopedia.wikia.com/wiki/Geologist#Geologists_and_Alcohol" target="_blank">alcoholic</a> <img src="../scipics/_ext.png"
/> .
.




 :
:Quote: Originally posted by bfesser  |
 . Perhaps it would be best if we split this
discussion out of Pretty Pictures and into a new topic on meteorites. Would that be alright with you?
. Perhaps it would be best if we split this
discussion out of Pretty Pictures and into a new topic on meteorites. Would that be alright with you?