blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Bonding in the nitrate ion
This question's been bugging me for a few days: Is the lone pair on the nitrogen atom in the nitrate ion involved in (coordinate covalent)
bonding to the third oxygen atom? Like:<tt>
- -
|·· ··| -1
| O::N:O:|
|·· · ··|
| · |
| :O: |
- ·· -</tt>
Where the rightmost oxygen atom is the one doing the coordinate covalent bonding. Sorry about the bad ASCII drawing.  I haven't been able to find any info about this. I haven't been able to find any info about this.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
looks ok to me. you will have an equilibrium of three states, however, all of which are identical - i.e. the extra negative charge on the right most
oxygen goes to the top and bottom ones -> electron delocalisation, making the whole thing more stable.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Actually it was the bottom oxygen atom that's supposed to be negatively charged, see the single bond there?  The rightmost oxygen atom has no charge, it's the one that coordinately
covalently bonded to the nitrogen via its lone pair. I already knew there was that bonding thingy going on where all bonds are identical and the
whole molecule carries that one charge, just it would be a <i><b>real</b></i> pain to draw a resonance diagram in ASCII. Btw,
my graphical pictures would look so much worse! The rightmost oxygen atom has no charge, it's the one that coordinately
covalently bonded to the nitrogen via its lone pair. I already knew there was that bonding thingy going on where all bonds are identical and the
whole molecule carries that one charge, just it would be a <i><b>real</b></i> pain to draw a resonance diagram in ASCII. Btw,
my graphical pictures would look so much worse! 
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I made a little drawing for you.
It also shows why nitric acid is a strong acid, because the nitrate ion has more resonance possibilities.
Acidic and basic behaviour can almost always be explained by resonance.
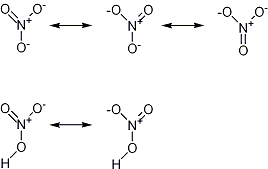
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Thanks for the pic, even though I've seen it before.
| Quote: |
Acidic and basic behaviour can almost always be explained by resonance.
|
Hmm, I didn't know that.  Cool. Cool.
|
|
|