MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Thermite Primer
Something I've been wanting to do for a while is write a primer on thermite reactions, to banish some misconceptions and gather everything I've
learned in one place for posterity. So here it is! I'll be posting this on my blog as well, and eventually turning it into a video on my YouTube
channel. Probably all of this information is on the forum someplace and many of you might know it all already, but in the spirit of aga's "SM Kemistry Skool" I thought I'd post it here anyway. Any corrections or recommendations are welcome.
========================================
Thermite: A Primer
What is Thermite?
Broadly, thermite is a solid/solid reaction between a powdered metal and a powdered metal oxide which is generally exothermic enough that the
products are in the molten state. They are also called Goldschmidt reactions or, when aluminum is the metal powder, aluminothermic reactions. The most
common formulation, and the most widely-known, is a 3:1 ratio of red iron oxide to aluminum powders. But this definition can encompass a very wide
variety of compositions, such as chromium, nickel, manganese, and silicon thermites. The metal powder can also be replaced with magnesium for more
stubborn reactions, for example enabling isolation of boron from boric oxide. Something else to note is that thermite reactions are, with one or two
exceptions, not explosive. They burn reasonably slowly at a very high temperature, and are better classed as incendiaries.
How Does it Work?
An example equation for a thermite reaction is the common version with red iron oxide:
Fe2O3 + 2Al == 2Fe + Al2O3
The metal oxide (rust) reacts with the metal powder (aluminum) to form aluminum oxide and a pure metal (iron). The oxygen essentially switches places
in a single displacement reaction. This also produces a tremendous amount of heat, and the products are formed in the molten state. When using
aluminum, this means that temperatures reached can reach or exceed 2,072 °C (3,762 °F), aluminum oxide’s melting point.
Somewhat counterintuitively, thermite compositions are very difficult to light. Even though they release a large amount of energy, it takes quite a
lot of energy to get them started (activation energy). Standard aluminum-rust thermite can be heated directly with a blow torch without igniting.
What is it Used For?
One of the most common uses for thermite is for welding in-place on large pieces of steel like railroad tracks, where transporting heavy welding
equipment is impractical. The intense heat produced can also cut through steel easily. A classic military use for thermite was to disable artillery
pieces by welding the loading or aiming mechanisms shut. Finally this class of reactions is also useful in isolating reasonably pure metals from their
ores.
Safety
Despite popular belief, thermite is not an explosive. It is more accurately classified as an incendiary, which burns rather slowly by comparison.
That being said, some formulations can be quite violent. Copper oxide thermite is known to react so quickly as to be near-explosive. Manganese
thermite produces gaseous manganese which could lead to an increase in pressure if confined.
The standard iron oxide thermite is a rather tame reaction, once one is familiar with it. A five-pound charge will burn completely through in roughly
10 seconds, accompanied by lots of sparks, smoke, and intense heat. Ensure this reaction is done well away from combustible materials (grass, leaves,
buildings, etc.) and anything wet or damp. Damp ground could lead to steam explosions when suddenly heated by molten iron. Also ensure that
participants and spectators are kept at least 15 feet away (depending on the size of the planned reaction). It is a good idea to place a layer of dry
sand on the ground around where the reaction will be done.
Ignition Strategies
Igniting a thermite mixture is very difficult. Despite the great amount of energy released during the reaction, it takes a very high temperature to
start it. Most normal ignition methods (matches, lighters, fuses, or even propane torches) will not ignite thermite. There are many different methods
that work; choose one that you are most comfortable with. This list is just a small selection; many more methods are available. My preferred method is
a combination of the first two listed here – see Tips below.
Magnesium ribbon – stick a strip of magnesium ribbon into the thermite pile, like a fuse, and light the ribbon with a
blowtorch.
Sparklers – stick a few sparklers into the thermite pile and light them at the top to form a fuse. This can be assisted by wrapping
some Mg ribbon around the sparklers as well.
Permanganate – add a small pile of potassium permanganate to the top of the thermite pile, then add a few drops of glycerin to this.
After a delay of 10 – 30 seconds, this will ignite and should in turn ignite the thermite.
Thermite Ignition Mix – some places sell specially formulated “ignition mix” that can be lit with a standard Visco fuse.
Tips for Making a Successful Thermite
Particle size: Smaller particle sizes lead to faster reaction rates; in some cases, a thermite will not work unless the
particle size is fine enough.
Thorough mixing: Solid/solid reactions are much more difficult to initiate than other types of chemistry, so intimate mixing is critical.
Ignition system: There are many different ways to ignite thermite, so use whatever you are most comfortable with. My preferred method is
complex, but has good reliability and does not require a lighter or blowtorch. I use a combination of the permanganate and magnesium methods: stick a
magnesium ribbon down through the permanganate pile so it extends down into the thermite mix but is still visible above the permanganate. See the
below graphic. When glycerin is added, the permanganate ignites which then ignites the magnesium which then ignites the thermite. The permanganate
reaction alone often isn’t hot enough to initiate the thermite on its own, and magnesium ribbon requires a blow torch to light and will often break
apart and go out before burning down to the thermite.
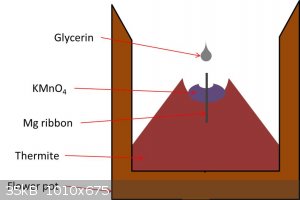
Procedure for 1 lb Standard Thermite
Weigh out 113.4g Al powder and 340.2g red iron oxide powder. A small excess of aluminum powder can help the reaction proceed.
Add both components to a plastic Ziploc bag, seal tightly and shake to mix thoroughly for several minutes.
Pour mixture into an appropriately-sized ceramic flower pot to form a conical pile. Place a piece of tape over the hole in the bottom of the pot
to keep the powder in.
Prepare the ignition system.
Dig out a small divot at the peak of the pile, to form a “volcano”.
Fill this divot with several grams of potassium permanganate.
Push a ~1” piece of magnesium ribbon into the permanganate pile, so that the ribbon extends down into the thermite mixture and also sticks out
the top of the pile.
For added spectacle, suspend the flower pot off the ground on a ring stand and position it over a bucket of sand. As the reaction proceeds,
molten iron will fall out the hole in the flower pot and collect on the sand.
When ready, add 2-3 drops of glycerin to the permanganate and step back. In 10 – 30 seconds, the ignition mixture will start to smoke. If all
goes well, this should catch fire, which should in turn ignite the magnesium ribbon which should in turn ignite the main thermite mixture.
After the Reaction
The products of this reaction are produced in the molten state. Ideally, they should separate cleanly and the metal will coalesce into large chunks
surrounded by a brittle shell of metal oxide. This depends on the relative densities of the products, however. A standard iron oxide thermite will
have excellent coalescence due to iron’s high density.
After allowing the products to cool, the results can be broken apart by lightly tapping with a hammer. In the case of the standard iron oxide
thermite, the aluminum oxide byproduct is brittle and can be easily broken. The metal product (in this case, iron) can be easily distinguished as
large chunks and/or small spherical beads. Also in the case of iron, it can be picked up and separated with a magnet.
Advanced Considerations
Some thermite compositions require more preparation, planning, and some extra ingredients to work best, depending on the experimenter’s needs.
Heat Boosters – These additives increase the running temperature of the reaction, by causing a side reaction that is
also exothermic. Simply, these run something along the lines of Oxidizer + Aluminum = Alumina + Byproduct + Heat. Therefore extra aluminum
needs to be added since some in consumed in this reaction. Heat boosters are needed for certain thermites where the main reaction does not produce
enough heat on its own to be self-sustaining, or to fully liquefy all the products. Some common heat boosters are calcium sulfate (gypsum, plaster of
Paris, drywall, etc.), potassium chlorate, and potassium nitrate.
Slag Fluidizers – These additives act as a flux, decreasing the viscosity of the molten products. This allows for better metal/slag
separation in the final product mass, yielding larger metal nuggets, better metal purity, and better metal recovery. One of the best slag fluidizers
is calcium fluoride (fluorite, fluorspar powder), but calcium oxide (quick lime) is also used.
Setup Considerations – The way a thermite reaction is set up depends on the goals of the experimenter. If this is being done for
demonstration purposes only, suspending the reaction vessel above a bed of sand is particularly flashy (see Step 5 above). But if one wants to collect
the products for study or element display, this is not ideal. To use thermite as a synthesis path, the most important consideration is metal/slag
separation. The reaction products must remain molten long enough for the metal to separate fully from the slag, and ideally coalesce into large single
nuggets. Heat boosters and slag fluidizers are important parts of this process, unless density differences are great enough to afford good separation
“naturally” (i.e. the standard iron thermite).
Further Reading
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Superb post MrHomeScientist !
EXACTLY what i was thinking of for SM Khemistry Skool.
Hang on - it's not in there ? Whu ?
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Wow, great contribution!
I also found this channel on youtube recently that shows a lot of nice experiments with thermite mixtures, the effects of fluxes, diferent oxides,
etc:
[part 1]: Fe, Cr, 304SS: https://www.youtube.com/watch?v=r0kfn4P2nfQ
[part 2]: Co, Ni, Sn, Mn, NiCr, FeMn: https://www.youtube.com/watch?v=pHWN6C4I664
[part 3]: Zr, V, Ti, Si: https://www.youtube.com/watch?v=LsdesMWC37g
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
RogueRose
International Hazard
    
Posts: 1596
Registered: 16-6-2014
Member Is Offline
|
|
Has anyone ever tried to ignite using nichrome or kanthal wire? both can reach about 1,400 °C (2,550 °F) before melting and you can do this with a
small coil with fairly low voltage (V depends upon length of wire).
have you found what temp is needed to ignite the mixture of iron thermite? Or possibly some sensitizer like a nitrate/chlorate (barium nitrate or
KClO3?) mixed at the point of contact with the nichrome wire?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I've heard of it being used but haven't tried it myself. It's worth looking into since you'd get good controllability of ignition timing. I imagine
the nichrome would be one time use!
|
|
|
|