DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
2-amino-3-nitrophenol synthesis - where to start??
In the distant future I want to attempt a Meerwein arylation, and for the final structure I am aiming at, I will need to start with
2-amino-3-nitrophenol:
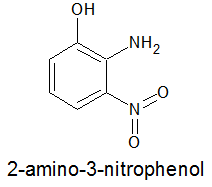
but I can't find a literature reference at any source I have access to. It's available commercially, and appears to have some history in hair dye
product etc.
Any suggestions?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
You can make it from 4-nitro-1,3-benzoxazole -https://www.heterocycles.jp/newlibrary/payments/form/05711/P...
if it is still available as a hair dye,that would be the most OTC way of getting it
What is the final structure you are aiming at ? Maybe an alternate starting compound could be suggested ?
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Are you certain this material can be successfully diazotized?
Somethings can be, some cannot.
And, since that phenolic hydrogen itself, might love to react with a diazonium salt, other couplings could be difficult.
Got a target molecule you might wanna share with us?
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Thanks for the response @CuReUs, but unfortunately with your referenced paper the 2-amino-3-nitrophenol is an input at the start of the reaction, it
is not synthesized as part of the procedure.
As far as @zed's comment, the basis for my question is the paper "Synthesis of Substituted Indoles via Meerwein Arylation" (Raucher, Koolpe, 1982 -
attached). Basically reaction couples vinyl acetate to a diazo'ed substituted benzene. Then there is a cyclization reaction step described that forms
an indole. With 2-amino-3-nitrophenol, the nitro part forms the -N- in the indole, and the -OH ends up in position 4 of the indole, which is tricky
with Fischer. That paper describes a reaction with a methoxy ending in the 4 position; yes, an -OH is probably trickier, but a protection group could
be used??
But first I have to make my 2-amino-3-nitrophenol...its a bit expensive to buy.
Attachment: raucher1983.pdf (600kB)
This file has been downloaded 432 times
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DrDevice  | | unfortunately with your referenced paper the 2-amino-3-nitrophenol is an input at the start of the reaction, it is not synthesized as part of the
procedure. |
it is made,but the yield isn't that great.Read the entire paper
| Quote: | | the -OH ends up in position 4 of the indole, which is tricky with Fischer. |
So basically you want to make 4-hydroxyindole.Why would it be tricky with fischer ?
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
OK, I think I see it now... The paper references other papers/methods/procedures that synthesize the 4-nitrobenzoxazole, that can then be reduced with
NaBH4/EtOH to form the 2-amino-3-nitrophenol. Hmm, projected yield is not exciting me.
So with the Fischer (courtesy Wikipedia):
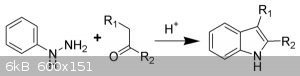
any substituent meta to the phenylhydrazine almost always ends up with much higher proportion in the 6 position rather than the 4 position of the
indole.
Other alternate approaches to 4-substituted indole such as "Useful synthesis of 4-substituted indoles" (Ponticello, Baldwin, 1979) also avoid the use
of Fischer.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DrDevice  | | OK, I think I see it now... The paper references other papers/methods/procedures that synthesize the 4-nitrobenzoxazole, that can then be reduced with
NaBH4/EtOH to form the 2-amino-3-nitrophenol. |
I am obviously not telling you to make 4-nitrobenzoxazole .What I had in mind was, you just might have some benzoxazole lying around which you could
reduce.4-nitrobenzoxazole is itself made from 2-amino-3-nitrophenol
why don't you try some other ways to make 4-hydroxyindole.Google turns up many erowid links.The regioselectivity of 2-amino-3-nitrophenol is difficult
to obtain.
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Well, I was thinking it was getting a bit circular there...
So yes, 4-hydroxyindole by other means. But the 2-amino-3-nitrophenol becomes a challenge. I have sort-of worked out a completely impractical sequence
from documented steps:
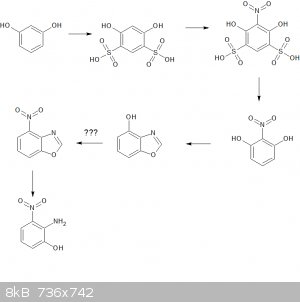
Start with resorcinol
Di-Sulfonate
Nitrate
De-sulfonate (the sulfonate-nitrate-de-sulfonate process is well documented)
Cyclize with triethylorthoformate (US Patent 4579865) to form 4-hydroxy benzoxazole
Then a miracle occurs  Maybe tosylate/halogenate/nitrate?? Maybe tosylate/halogenate/nitrate??
Reduce with NaBH4/EtOH as per previous paper.
Et voila!
So like I said, maybe impractical 
But is there perhaps another starting phenol but with a different substituent in the 2- position that would still be di-sulfonated, driving the
subsequent nitration to the desired position, then de-sulfonated?
Followed by reduction of of the nitro to an amine, then convert substituent to nitro?
OR is there a feasible methodology to substitute/protect just one of the -OH in the 2-nitroresorcinol?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
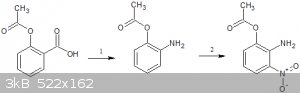
you could make O-acetylamino nitrophenol from aspirin
1.schimdt reaction on aspirin to get O-acetylamino phenol
2.o-nitration using amyl nitrate(pg 2977) -http://pubs.rsc.org/en/Content/ArticleLanding/1954/JR/jr9540...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Retrosynth:
Elbs persulfate oxidation of 2-nitroaniline:
http://en.wikipedia.org/wiki/Elbs_oxidation
Ullmann reaction of 2-chloronitrobenzene and ammonia; nitration of chlorobenzene and separation of isomers. Or nitrodecarboxylation of 2-chlorobenzoic
acid from rxn of hypochlorite with sodium benzoate (alkaline conditions give 2-isomer)
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
I think you've solved it 
Following the references to the Elbs oxidation, I found references to the Boyland-Sims oxidation and the paper "The Oxidation of Some Aromatic Amines
with Persulphates" (Boyland, Sims, 1954, copy attached) with the desired 3-nitro-o-aminophenol as one of the examples.
And I have all the required reagents, so a project for over Christmas holidays.
Thanks again for the pointers!
Attachment: boyland1954.pdf (681kB)
This file has been downloaded 430 times
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummmm. Them little mushrooms are native to my area. And, we consider them legal. More or less. Pop-up on the lawns, at Reed College.
So a walk in the park, seems more practical, than a trip to the lab.
That being said, a few years back, some fellas concocted a lab synthesis, that was pretty straightforward looking.
https://erowid.org/archive/rhodium/chemistry/psilocin_synthe...
Instead of starting from an exotic ring structure, they convert ordinary indole into its 3-aldehyde. Which seems to activate the 4 position... Etc.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So I just read the threadstarter -- do Meerwein arylations work on o-hydroxyanilines? It seems like you'd get some weird diazo-quinone thing. You
might want to make an O-protected version -- luckily the phenol should be much more reactive towards substitution than the aniline, so I think you'd
get a good yield with stoichiometric benzyl chloride at low temps.
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
O-protection thoughts were next on the agenda.
The source paper for the approach is "Synthesis of Substituted Indoles via Meerwein Arylation" (Raucher, Koolpe, 1983, attached). There were only a
limited number of substituents attempted, and -OH wasn't one of them.
Using this approach, it would need to be a fairly funky substituted alkene to end up with an ethylamine in the right place for this reaction to end up
with anything, um, dodgy.
Attachment: raucher1983.pdf (600kB)
This file has been downloaded 994 times
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | So I just read the threadstarter -- do Meerwein arylations work on o-hydroxyanilines? It seems like you'd get some weird diazo-quinone thing. You
might want to make an O-protected version |
hence my aspirin approach  -http://www.sciencemadness.org/talk/viewthread.php?tid=78901#... -http://www.sciencemadness.org/talk/viewthread.php?tid=78901#...
In case you successfully make the vinyl nitrophenol ,here is another ref for the cyclisation -http://pubs.acs.org/doi/10.1021/jo970516%2B Quote: Originally posted by zed  | | Instead of starting from an exotic ring structure, they convert ordinary indole into its 3-aldehyde. Which seems to activate the 4 position... Etc.
|
That's what even I suggested upthread, since there is a way to convert indole directly to 4-hydroxyindole.But maybe the OP has a problem procuring
indole ?
|
|
|