mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
coumarin 7 synthesis
Hi im interested in making some coumarin 7 as a chemiluminescent dye (it gives green color which is quite rare) but i cant find even informations
about this compound not to mention synthesis. It might be because i only search on internet because i dont have access to chemical literature. I only
have its formula from sigma aldrich. Can someone help how to synthesise it easiest way and without using too exotic chemicals?
This is its formula:
http://www.sigmaaldrich.com/content/dam/sigma-aldrich/struct...
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Interesting structure but this is no simple synthesis.
My proposed route:
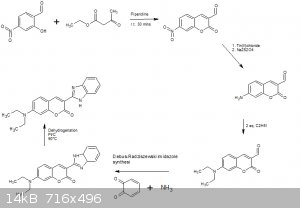
I must admit though i broke a few of your rules 
The first reaction between 4-nitro-salicylaldehyde and ethyl acetoacetate was taken from the following:
https://www.omicsonline.org/open-access/synthesis-and-trypan...
Preparation of 3-acetylcoumarin (1) [22]: A mixture of salicylaldehyde (1 eq.), ethyl acetoacetate (1 eq.) and a few drops of piperidine were
mixed for 30 min. at room temperature without any solvent. Reaction was neutralized with HCl (dil.) and finally the product was isolated by
filtration. The final compound was then recrystallized in EtOH.
Don't ask me how to prepare 4-nitro salicylaldehyde. If i find something ill bring it back here.
2nd step would be a basic nitro reduction and sodium dithionite would certainly be ideal for that, i think its usually used as 3 times equivalent to
the reduced substrate.
3rd step is again a self explanatory, alkylation of the amine using haloethane, i used iodoethane in my sketch.
The 4th step calls for the use of 1,2-benzoquinone which could be easily prepared via oxidation of catechol.
The dehydrogenation is self explanitory but read here for a basic overview:
http://www.chemgapedia.de/vsengine/vlu/vsc/en/ch/2/vlu/oxida...

The only major issue i can see is the use of 4-nito salicylaldehyde which is not cheap or easy to find and buy or easy to prepare but the rest of the
reagents can be acquired reasonably easily and i think a prep for all of them would exist on the forum somewhere if you need aid with these, i may
even be persuaded to point you in the right direction.
This synth may be flawed but it will atleast break the ice and encourage others to put forward their ideas.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Thank you very much now i have straight way how to do it i just have to prepare ingredients. It isnt so hard to make i think since it doesnt need
exotic catalysts or working under pressure in different gases atmosphere.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
fish,you made a mistake in the first step,the condensation forms a ketone,not an aldehyde -https://www.omicsonline.org/articles-images/medicinal-chemis...
Mack ,see this-http://www.tandfonline.com/doi/full/10.1080/0039791090302669...
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Although that route looks like it should work, it's not going to be clean. I can't speak for the first reaction, as I am not familiar with it. Looking
at the reduction, it would probably be best to avoid using sodium dithionite, because when it is oxidized, sodium bisulfite is produced as a
byproduct, which could participate in a side reaction with your aldehyde. The alkylation of the amine is problematic as well because even if you only
use two equivalents of ethyl iodide, you'll inevitably get some overalkylation to the quaternary ammonium compound, and some underalkylation to make
up for it. So then you have a lovely mixture to separate and will probably take a hit on the yield. Also as pretty as that dehydrogenation looks,
knowing palladium, there's a good chance it won't be that selective.
I'm not saying it won't work, definitely try it, but be cautious about it. Test each reaction on a small scale before jumping in.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
fish,you made a mistake in the first step,the condensation forms a ketone,not an aldehyde
|
Damn, i was so confident too.
Still there are other ways working with a similar reaction to get to other workable products, maybe even possible to work with
1-Ethoxy-2-butanone-4-al instead of the acetoacetate but that might be a bit tricky to aquire. I dont have full access to your ref but it looks like
it may solve the imidazole problem without the need for an aldehyde, maybe.
| Quote: |
Also as pretty as that dehydrogenation looks, knowing palladium, there's a good chance it won't be that selective.
|
Pretty sure it takes place using platinum not palladium, again probably not very selective but i cannot see any possible side reactions that would
take place.
Edit: just thought of a way to solve the messy alkylation of the amine, we deliberately over alkylate to the quarternary amine using 3eq of ethyl
halide and then simply react that with a base to freebase the amine back to a tertiary.
[Edited on 2-11-2017 by Assured Fish]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I would be pretty tempted to attempt the installation of the benzimidazole as the final step and without changing the oxidation state of the number 2
carbon of the benzimidazole. That is; directly from o-Phenylenediamine and a carboxylic acid, an ester or a nitrile. After searching, someone has
indeed published about this in Journal Synthetic Communications, Volume 40, 2010 - Issue 6, Microwave-Promoted One-Pot Syntheses of Coumarin Dyes
Farahnaz Nourmohammadian & Mahnaz Davoodzadeh Gholami.
http://www.tandfonline.com/doi/full/10.1080/0039791090302669...
my proposed route: now I know that the starting aldehyde is commercially available quite cheaply but it can after all be made.
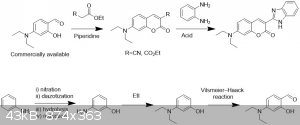
Edit: fixed link
[Edited on 3-11-2017 by Sigmatropic]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Sigma but what for is o benzyl diamine for since 1,2 benzochinone is easier to get.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
And wouldnt it be easier to make m aminophenol starting from phenol or resorcinol? I read that resorcinol substitution reaction requires autoclave. Is
there any other way to make it from phenols? Maybe making it via hoffman rearrangement maybe reducing amide to amino group
[Edited on 3-11-2017 by mackolol]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
3-aminophenol from phenol wouldn't be easy because phenol is o, p directing. For that matter, so is aniline, so the nitration that Sigmatropic
suggests would not be effective. You'd need to start with (or make) benzamide, nitrate that to 3-nitrobenzamide, and then perform a Hoffman
degradation to 3-nitroaniline. which could then undergo the rest of the process described. Nevertheless, it's even longer and messier.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
zts, remember that aniline is protonated under conditions of nitration. Nitration of aniline affords m-nitroaniline. nitration of acetanilide is o,
p-directing. Another route to 3-aminophenol is sulfonation of nitrobenzene, hydroxylation in molten alkali and reduction of the nitro group.
surely the 2 step process is not longer than the route proposed by assured fish. The starting point of that synthesis is not trivial either.
[Edited on 2-11-2017 by Sigmatropic]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
As it would seem the precursor 1-ethoxy-1-propanon-3-al may be easier to prepare than i first thought.
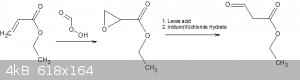
This would be rather simple starting from ethyl acrylate which is used for the production of several polymers and resin and according to sigma as a
flavoring agent.
http://www.sigmaaldrich.com/catalog/product/aldrich/w241806?...
The stuff would stink to high heavens would burn your eyes and lungs and it would also polymerize without an inhibitor but its workable within a fume
hood or with a good respirator.
Pretty cheap too if you can find a good source.
I should also point out that it may be possible for H2SO4 to rearrange the oxide to the aldehyde given the steric hindrance of the ketone in close
proximity.
https://erowid.org/archive/rhodium/chemistry/meinwald.rearr....
Edit: sigma your ref doesn't work http://www.tandfonline.com/doi/abs/10.1080/00397910903026699...
[Edited on 3-11-2017 by Assured Fish]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  | | we deliberately over alkylate to the quarternary amine using 3eq of ethyl halide and then simply react that with a base to freebase the amine back to
a tertiary. |
you cannot convert a quat to a tert like that  .You would have to do the Emde
degradation ,which would then make the synthesis redundant -https://en.wikipedia.org/wiki/Emde_degradation .You would have to do the Emde
degradation ,which would then make the synthesis redundant -https://en.wikipedia.org/wiki/Emde_degradation
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by Sigmatropic  | zts, remember that aniline is protonated under conditions of nitration. Nitration of aniline affords m-nitroaniline. nitration of acetanilide is o,
p-directing. Another route to 3-aminophenol is sulfonation of nitrobenzene, hydroxylation in molten alkali and reduction of the nitro group.
surely the 2 step process is not longer than the route proposed by assured fish. The starting point of that synthesis is not trivial either.
|
Oh damn, I forgot about that. Yeah I guess that nitration would work alright.
And I wasn't saying that your path is longer than AssuredFish's, just that either way when you include the synth of the starting material it's quite a
long synthesis overall, and not suitable for someone with limited organic synthesis experience.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
May I suggest instead the synthesis of fluorescein and eosin for people limited in materials and equipment.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
you cannot convert a quat to a tert like that  .You would have to do the Emde
degradation ,which would then make the synthesis redundant -https://en.wikipedia.org/wiki/Emde_degradation .You would have to do the Emde
degradation ,which would then make the synthesis redundant -https://en.wikipedia.org/wiki/Emde_degradation
|
Seems i thought refluxing with a base was sufficient to dealkylate.
Though this makes me wonder if a protinated amine can still be alkylated to a tertiary without going all the way to quarternary.
found the following which uses a alkyl bromide and HBr to protonate so as to avoid neucliophilic substitution i think, but it says nothing about going
from secondary to tertiary only primary to secondary.
http://pubs.rsc.org/-/content/articlelanding/2014/ra/c4ra019...
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
So... It won't work?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So resorcinol is a substrate for the Bucherer reaction:
http://en.wikipedia.org/wiki/Bucherer_rearrangement
This means that transformation of resorcinol to 3-aminophenol is possible. Reductive alkylation with acetaldehyde gives 3-N,N-diethylaminophenol.
Formylation should go primarily to 2-formyl-5-N,N-diethylaminophenol. This can then be transformed to 7-diethylamino-3-carboxycoumarin by the action
of malononitrile in water:
http://courses.chem.psu.edu/chem431/800Expts/864.pdf
This rxn product then reacts with o-phenylenediamine to afford the desired coumarin.
In order to synthesize o-phenylenediamine, there is one patent which begins with p-dichlorobenzene, nitrates this, reacts with ammonia to
2-nitro-4-chloroaniline, and hydrogenates that to o-phenylenediamine. This is posted in the thread about para-dichlorobenzene, which is the principle
odorant in mothballs.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
But why iodoethane won't work?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It could work. Quaternization depends on the solvent used and some other factors. Quaternization of anilines is usually pretty slow. I was more
focused on the other reactions when I wrote that post.
I had a friend who had good results producing a tertiary di-ethylated amine from a primary amine using EtBr+Na2CO3 in EtOH. I think that with more
strongly dipolar solvents (acetone, acetonitrile, DMF etc) you'll see more quaternization but not as much in EtOH.
But the bigger problem with alkyl halides is alkylation of the phenol. I don't know if you can avoid this as easily when alkylating 3-aminophenol.
Although acetaldehyde also bears the risk of ring-alkylation.
Anyway my main contribution I think is that malononitrile can be a more stable/easily handled reagent than formylacetate esters.
[Edited on 4-11-2017 by clearly_not_atara]
|
|
|