| Pages:
1
2 |
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Novel route to Methylone?
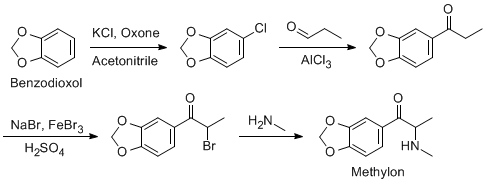
Hi there...
I've really set my mind on a Methylone synthesis, since all reagents and precursors are widely available.
I know that the first, third and fourth step gives near quantitive yields, but i don't know if it's possible to condensate propanal like that in
second step.
- That's why i'm asking here. 
AFAIK the oxygen should be a good electrondonor, thus making the nearest hydrogen most willing to leave.
Am i totally wrong about this? Maybe with the help of another catalyst, could this be possible in good yields?
I know that propionyl chloride can be used in the first step, but i would really like to avoid that one.
Thanks in advance
Dex
[Edited on 13-1-2007 by Dextrose]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Yet another flavor & fragrance chemist.
The step you propose is a Friedel-Crafts. This ought to be in the literature, at least for chlorobenzene.
See if the 3,5 methylenedioxy group will affect the F-C one way or the other.
[Edited on 13-1-2007 by Sauron]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That step is not a Friedel-Crafts reaction. Actually it has no name since it is complete nonsense. A Friedel-Crafts reaction is an
electrophilic substitution of a hydrogen atom on an nucleophilic aromatic compound * by a strong enough electrophile generated by the
complexation of a Lewis acid catalyst with an otherwise too weak electrophile. The main (and perhaps the only) difference between a Friedel-Crafts
reaction and the numerous other electrophilic aromatic substitutions is that the electrophile is a carbocation.
Dextrose you might start by reading some books on basic organic chemistry first (before planning syntheses) and preparative chemistry (before actual
experimentation). But first read about the Friedel-Crafts reaction as well as it seems you have lots of confusion regarding it and also a complete
lack of insight in its mechanism:
http://en.wikipedia.org/wiki/Friedel-Crafts_reaction
http://www.chemguide.co.uk/organicprops/arenes/fc.html
http://www.organic-chemistry.org/namedreactions/friedel-craf...
http://www.organic-chemistry.org/namedreactions/friedel-craf...
* Which is not the case in your proposal.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
My suggestion in sending him to study the F-C rxn was for him to discover on his own that the -Cl was not needed.
Better pedagogically for him to learn on his own. And he had his heart set on using AlCl3.
But anyway now that is short circuited. Oh well.
I do believe the appropriate F-C acylating agent to be propionyl chloride not propanal. And the target aromatic the methylenedioxy benzene not the
corresponding chlorobenzene.
That might be feasible. Cf. the well known prep of chloroacetophenone from benzene and chloroacetyl chloride over AlCl3.
I have not looked up F-C acylation yet so I can only speculate about what effect the presence of the phenolic ether functionaility might have. That's
a mildly activating group insofar as Hammett is concerned, rather like anisole. To go beyond that comment I would have to crack a book.
[Edited on 13-1-2007 by Sauron]
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
Aluminum chloride is incompatible with methylenedioxy bridges, since the result will be polymerization  See Khim.Farm.Zh.; RU; 21; 5; 569-573 (1987) for a feasible procedure See Khim.Farm.Zh.; RU; 21; 5; 569-573 (1987) for a feasible procedure 
Chemistry is our Covalent Bond
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, leu. I think I'll let someone else dance with this one, it really does not interest me much.
A quick look at an old review of F-C suggested that phenols were a complex substrate because of possible coordination between the oxygen (or oxygens)
and the Lewis acid.
Still there were some examples of succesful alkylations of anisole.
Perhaps not with AlCl3.
I don't read Russian anyway, although I suppose they have drawn some rxn schemes.
But thanks again.
[Edited on 13-1-2007 by Sauron]
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I know that propionyl chloride can be used in the first step, but i would really like to avoid that one. |
As i wrote, i would like to avoid F/C, since i don't like acyl-/alkyl-halides.
The question was, is this condensation possible with any catalyst at all?
Thanks.
I've read heavily up on my McMurry, Carey, etc. in the past months, so please don't ask me to plow more books.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Dextrose
The question was, is this condensation possible with any catalyst at all? |
I thought it was obvious the answer was "No"?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I believe Nicodem called that "condensation" complete nonsense.
There's your answer. I won't quarrel with him.
I think you had best plan on ploughing through a lot more books. A few undergrad tests for a few months? I've been at this for 40 years and I still
plough through a lot of books. We call that using the chemical literature, and a chemist regards it as home and hearth and mother's milk.
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nicodem
| Quote: | Originally posted by Dextrose
The question was, is this condensation possible with any catalyst at all? |
I thought it was obvious the answer was "No"? |
No it wasn't obvious to me, but if the answer is a no, i would like to hear your argumentation why, so i can increase my knowledge.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@leu was kind enough to point out why AlCl3 is not the way to go and to cite a reference that gives a feasible procedure.
Khim.Farm.Zh.; RU; 21; 5; 569-573 (1987)
Go to the Wanted References and Translations thread in References forum, obtaining a PW if you don't have one. Ask politely for help in obtaining that
article and if it in Russian language as seems probable, ask for a translation.
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Thank you.
I must admit that AlCl3 as catalyst was just a wild guess, but i had no other ideas.
Will aquire the reference, ask politely ( ) and study some more. ) and study some more.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
A wise plan of action.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Dextrose
No it wasn't obvious to me, but if the answer is a no, i would like to hear your argumentation why, so i can increase my knowledge.
|
Because there is no viable mechanistic explanation for such a condensation. And besides, propanal in the presence of AlCl3 immediately creates tar due
to the alpha-hydrogens condensing with the aldehyde group. Even if an aldehyde without alpha-hydrogens would be used, for example benzaldehyde, one
would utmost obtain only the Friedel-Crafts reaction product, namely the SMILE code Clc1cc5OCOc5cc1C(c2cc3OCOc3cc2Cl)c4ccccc4
in the case of 6-chloro-1,3-benzodioxole and benzaldehyde.
| Quote: | Originally posted by leu
Aluminum chloride is incompatible with methylenedioxy bridges, since the result will be polymerization |
Actually, according to Kinetic, AlCl3 can be used in the Friedel-Crafts reaction of benzodioxole with Et-COCl if nitromethane is used as a solvent at
0°C. The yield was a bit on the low side (40%), which is however still very much better than the complete cleavage that happens in CH2Cl2, for
example. In my personal opinion the same yield might be obtainable by using propanoic acid in polyphosphoric acid, but such Friedel-Crafts acylation
is a bit tricky and a good strength polyphosphoric acid must be used (pyrophosphoric acid made from H3PO4 only gives a terribly low yield on a
substrate similarly reactive as benzodioxole).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ullmann
Hazard to Self
 
Posts: 51
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
There is a nice paper about FC reaction using the free acid and carbon/p-toluenesuphonic acid as catalyst and reactant... it is quite new and
published in thieme synthesis... there is no need for the acyl chloride nor for polyphosphoric acid in this paper...
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Citation please for the sake of those who want to look this up.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I think Ullmann is refering to Helvetica Chimica Acta, 88(8 ), (2005), 2282 - 2287. If there is really a similar paper in
Synthesis, I'm unaware of it. The referred paper can be found in the old Wanted References and Needed Translations (about in the middle of the thread, requested by Vitus and uploaded by Mephisto). However, the authors
have not included any examples using benzodioxole. Nevertheless it just might survive the conditions of 17mol% TsOH at 90°C that they use.
Seems like benzodioxole can survive even the toughest protic acids as long as they are in catalytic amounts only. For example, in US6342613 they condense propionic anhydride with benzodioxole with perchloric acid as Friedel-Crafts catalyst at room temperature. That is only
2.6mol% of catalyst and at a lower temperature, but nevertheless the acid is added to plain benzodioxole at 0°C before the addition of the anhydride.
The yield is only 36%, but the unreacted benzodioxole gets recovered.
Edit:
I checked Synthesis for any papers using graphite and a protic acid for the Friedel-Crafts acylation and could only come out with two papers from the
same Iranian authors as for the Helv. Chim. Acta paper. In both papers they use the less accessible and more expensive methanesulfonic acid in a much
higher mol%. One from 2005 is about the acylation of phenols, but an older one from 2004 (attached bellow) is about phenol ethers acylation. The paper must
have never seen any peer reviewer as it is full of terrible errors (just look the last two entries in Table 2!). Just another blatant example of how
the peer reviewing system slowly decays dragging the academic science with it. Now I have serious doubts about their method especially since they use
way more methanesulfonic than they later use tosylic acid on same substrates at the same conditions. But most annoying is the presence of the entry 8
in Table 2 with the 2-pyridinecarboxylic acid which would definitively neutralize/quench the 50mol% MeSO3H used as catalyst! Looks like Synthesis is
getting as shitty as Tetrahedron Letters!
[Edited on 14-1-2007 by Nicodem]
Attachment: s-2004-831162.pdf (588kB)
This file has been downloaded 3590 times
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
To go back to the first post: it may be possible to react the organomagnesium compound derived from 1-halo-3,4-methylenedioxybenzene with
propionitrile. Hydrolysis of the thus formed iminium salt yields the corresponding propiophenone.
damnant quod non intelligunt
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Calling Dr Grignard!
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
what about ketonization of piperylic acid with propionic acid?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
I once posted the paper mentioned above by Nicodem and Ullman to the russian HyperLab forum, below follows experimental procedure:
---------------------------------------------------------
Speaking of arylketones...
Solvent-Free Catalytic Friedel-Crafts Acylation of Aromatic Compounds with Carboxylic Acids by Using a Novel Heterogeneous Catalyst System:
p-Toluenesulfonic Acid/Graphite
Sarvari, Mona Hosseini; Sharghi, Hashem. Chemistry Department, College of Sciences, Shiraz University, Shiraz, Iran. Helvetica Chimica Acta (2005),
88(8), 2282-2287.
Abstract
TsOH/graphite was found to be an effective catalyst system for the Friedel-Crafts acylation of aromatic compounds with carboxylic acids. Both
aliphatic and aromatic carboxylic acids reacted smoothly under TsOH/graphite catalysis to afford the corresponding aromatic ketones in high yields.
The graphite was easily recovered by simple extraction and could be reused without decrease of activity in the presence of fresh TsOH.
Acylation of Aromatic Compounds, General Procedure: To a mixture of graphite (CAS No.7782-4 2-5; fine powder extra pure, from Merck; 0.3 g, 25 mmol)
and anh. TsOH (0.03 g, 0.17 mmol) at 90 C in an oil bath the aromatic compound (1 mmol) and carboxylic acid (1 mmol) were added. The mixture was kept
at 90 C while stirring with a mechanical stirrer for the time required to complete the reaction (see Table 2; TLC or GC monitoring). The solid mass
(graphite) was then extracted with AcOEt (20 ml) and the AcOEt extract washed with aq.NaHCO3 soln., dried (Na2SO4) and evaporated: practically pure
product. Yields are excellent.
What a lazy referee at Synthesis missing such obvious errors...  
| Quote: | Originally posted by Sergei_Eisenstein
To go back to the first post: it may be possible to react the organomagnesium compound derived from 1-halo-3,4-methylenedioxybenzene with
propionitrile. Hydrolysis of the thus formed iminium salt yields the corresponding propiophenone. |
I have posted a paper (Org. Lett IIRC) in which a method is presented that involves the preparation of arylzinc reagents from elemental Zn (dust) in
dimethylacetamide (I2 catalyzed), these reagents react with acylhalides (I guess anhydrides would also work) to give corresponding phenones
(one-pot)... Relevant thread can be found using tfse (Sandmeyer Knochel).
[Edited on 16-1-2007 by Sandmeyer]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Sandmeyer
Solvent-Free Catalytic Friedel-Crafts Acylation of Aromatic Compounds with Carboxylic Acids by Using a Novel Heterogeneous Catalyst System:
p-Toluenesulfonic Acid/Graphite
|
bump! just one thought...reacting benzodioxole with a carboxylic acid the way they propose it in the above mentioned paper, i wonder if there is ever
any ortho-acylated product formed?
what about acylation of benzofuran, would it be acylated next to the oxygen?
this method looks very good on paper, if you have Pd/C, DMF and POCl3, you're set for yet another easy route to the 2C-X series.
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | | this method looks very good on paper, if you have Pd/C, DMF and POCl3, you're set for yet another easy route to the 2C-X series.
|
If you have access to Pd/C, DMF and POCl3, all routes to 2C-X are easy.
| Quote: | | reacting benzodioxole with a carboxylic acid the way they propose it in the above mentioned paper, i wonder if there is ever any ortho-acylated
product formed? |
It may be possible that some o-acylated product is formed, but this is not the main route. If you desire to synthesize o-acylated
methylenedioxyarenes, you can use organolithium chemistry.
damnant quod non intelligunt
|
|
|
Epopteia
Harmless

Posts: 8
Registered: 19-7-2005
Member Is Offline
Mood: Yes
|
|
nitromethane co-ordination of AlCl3
| Quote: | | Actually, according to Kinetic, AlCl3 can be used in the Friedel-Crafts reaction of benzodioxole with Et-COCl if nitromethane is used as a solvent at
0°C. The yield was a bit on the low side (40%), which is however still very much better than the complete cleavage that happens in CH2Cl2, for
example. |
His procedure did indeed work, but the workup is a nightmare, and I think a lot of losses happen there.
If a better workup was found it could be possible to do much better.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Sergei_Eisenstein
If you desire to synthesize o-acylated methylenedioxyarenes, you can use organolithium chemistry. |
actually, i'd prefer the para-acylated product, so this procedure might be worth a try.
about organolithium chemistry...i don't feel to comfortable with BuLi, it scares me just as high pressure cat.hyd. does. are my fears justified?
|
|
|
| Pages:
1
2 |