| Pages:
1
2 |
Elawr
Hazard to Others
  
Posts: 174
Registered: 4-6-2006
Location: Alabama
Member Is Offline
Mood: vitriolic
|
|
Chromium and nickel separation
I recently dismantled my old Paragon ceramic kiln intending to salvage the refractory bricks and electrical parts. The steel bottom of this ancient
kiln had rusted out, and the heating elements were badly imbrittled, so much so, that some of the nichromes wires snpped like strands of spaghetti
when bent. The salvage operation yeilded a susbstantial mass of friable and brittle heating wire that seemed of little further use as far as
electrical resistance heating is concerned. Discarding the wire was of course also out of the question. Surely with my knowledge of chemistry I could
turn this into something wonderful. Therefore about 500cc 27% HCl was set to work, making fairly quick work of the metal over several days. What I
ended up with was a vat of wickedly dark blue/black to green solution somewhat reminescent of chrome alum. A veritable witches brew of toxic heavy
metal!
I suspect what I have is a mixture of aqueous Cr(III) and Ni(II) as aqueous complexes with Cl-. It would be fun to separate and isolate these two
metals using the limited technology I have here in my garage, (i.e baking soda, vinegar, hotplate, etc.).
Chromium should be the more acidic in solution, so nickel should tend more to be thrown down as the carbonate at some point, leaving Cr(III)
dissolved. My first experiment along this angle titrating with NaHCO3 so far has produced no precipate that I can for sure say to be NiCO3.
Any thoughts, anyone?...
[Edited on 21-10-2006 by Elawr]
1
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Basify with a excess of base. Ni(OH)2 will form and precipitate, wheras the chromium will form its hydroxide and then dissolve in the base forming
chromite(?). The Ni(OH)2 can be filtered out, and then the chromite solution can be acidified to either chromium hydroxide, or right past it to a
soluble salt.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Nickel carbonate dissolves in excess K2CO3.
Adding aluminium sulfate and then using aqueous ammonia in excess will precipitate the Cr and Al, and pull the Ni into a solution as a complex.
|
|
|
unionised
International Hazard
    
Posts: 5134
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Why add aluminium?
Slow addition of NaOH should ppt the Cr(III) first. If the colour change is obvious enough you might get quite a good separation this way (I have done
this with Fe+++ and Nd+++).
Adding ammonium sulphate might bring the Cr out as chrome alum but that will only work if the Cr is present as the hexaquo comlex. With all that
chloride there I think that it won't work as well. (Cr(III) complex equilibria are slow).
Ni will not disolve in excess OH-; Cr(III) will- it will do so more readily if there's an oxidant present to turn it into chromate but you might not
want to generate carcinogenic materials more often than you need.
Both Cr(III) and Ni(II) are soluble in ammonia (Cr less so than Ni) so I don't think that will give a very good separation.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Maybe you'd like to try heating with excess NH4Cl, with NH4OH [don't start...] added slowly to the HOT solution just until the smell of ammonia
remains. The precipitation of Cr(OH)3 and complexation of Ni in solution is supposed to be OK on analysis; as long as the excess of NH4OH is small and
that of the NH4Cl isn't. Big difference in adding ammonia to Cr+3 hot and cold.
The high temperature exposure and washing with HOT dilute NH4Cl instead of water also gives a slightly more filterable (add cold Na2CO3 to CrCl3 and
wash with water to see what fun Cr(OH)3 is) precipitate. Not that there is anything wrong with decanting IMHO.
I suppose that if there is already excess HCl in this mixture, it could just be a matter of adding enough ammonia then.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Cr(III) tends to incompletly ppt as the hydroxide, a fair percentage stay as collodial solution, unless there's something else dropping out at the
same time. Aluminiumworks well, and isn't difficult to separate from the chromium afterwards.
Ni(II) seems to complex with ammonia more readily than Cr(III), if oxidation is avoided it is a reasonable first cut separation.
Note that some heater wire also contains iron, if you don't know the exact composition of yours then expect some Fe to be there.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Hello
Bringing up this old thread since I got a roll of over 2 kg of NiCr wire and because I dont have any nickel salts I want to extract some of it to make
a small amount of nickel sulfate.
The_Davster in this thread and someone in another thread said that because of the amphoteric nature of chromium oxide will dissolve in excess of
base, so my plan is to dissolve some (about 50-100g) in HNO3, aqua regia or HCl/chlorate and precipitate all with NaOH, wash with water then leach
with hot concentrated NaOH to dissolve chromium oxide.
Could this work well?
Thanks!

"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Aqua_Fortis_100%  | Hello
Bringing up this old thread since I got a roll of over 2 kg of NiCr wire and because I dont have any nickel salts I want to extract some of it to make
a small amount of nickel sulfate.
The_Davster in this thread and someone in another thread said that because of the amphoteric nature of chromium oxide will dissolve in excess of
base, so my plan is to dissolve some (about 50-100g) in HNO3, aqua regia or HCl/chlorate and precipitate all with NaOH, wash with water then leach
with hot concentrated NaOH to dissolve chromium oxide.
Could this work well?
Thanks!
|
Should work. Perhaps even better if in strongly alkaline conditions you kick the Cr(+3) to CrO4<sup>2-</sup>
(chromate) - Cr(+6)) with H2O2, then separate Ni(OH)2 and sodium chromate by filtering and washing.
Alternatively, precipitate the hydoxides, wash carefully and redissolve in 37 w% HCl. CrCl3 is far more soluble than NiCl2, so
fractional crystallisation, seeing also that Cr is the minority constituent, shoud yield relatively pure NiCl2.
[Edited on 16-2-2016 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Tdep in his YT channel extractions and ire uses calcium hypochlorite. It oxidises Cr(III) to CXr(VI) and calcium chromate precipitates out.
His starting material is stainless steel forks so not much nickel. But it should work the same. Also he is after the chromium rather than the other
elements.
Next attempt of mine to separate Cr and Ni, I will go that route.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Tdep in his YT channel extractions and ire uses calcium hypochlorite. It oxidises Cr(III) to CXr(VI) and calcium chromate precipitates out.
|
I see a problem there: that oxidation requires alkaline conditions. Precipitating CaCrO4 then presents no advantage, as Ni(OH)2
is also insoluble.
[Edited on 16-2-2016 by blogfast25]
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ok then. I will need to take a closer look at what he actually did. I am going from memory.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4403
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by blogfast25  |
Should work. Perhaps even better if in strongly alkaline conditions you kick the Cr(+3) to CrO4<sup>2-</sup>
(chromate) - Cr(+6)) with H2O2, then separate Ni(OH)2 and sodium chromate by filtering and washing.
|
This is how the two metals are separated in classical qualitative analysis.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
CaCrO4 has decent solubility in water, so you can seperate it pretty easily from the Ni(OH)2. The chromate then crystallises out first if the chromate
solution is boiled down, before any calcium chloride or chlorate impurities.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tdep  | | CaCrO4 has decent solubility in water, so you can seperate it pretty easily from the Ni(OH)2. The chromate then crystallises out first if the chromate
solution is boiled down, before any calcium chloride or chlorate impurities. |
That explains the above then. Thanks!
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
I would be inclined to add excess ammonia. Nickel forms a soluble ammine. I don't think Chromium does so will not dissolve.
If you're not part of the solution, you're part of the precipitate.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4403
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by nezza  | | I would be inclined to add excess ammonia. Nickel forms a soluble ammine. I don't think Chromium does so will not dissolve. |
Chromium forms a wide variety of ammonia complexes, but conversion from one to the other is slow. This will muck up any attempt at separation.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Thanks for the info Draconic acid. I have never been able to get Chromium III to dissolve in ammonia. One other reaction where the Chromium salt is
insoluble and the nickel salt soluble from my experience is chromate. Nickel chromate appears soluble and chromium III chromate is insoluble.
If you're not part of the solution, you're part of the precipitate.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by nezza  | | One other reaction where the Chromium salt is insoluble and the nickel salt soluble from my experience is chromate. Nickel chromate appears soluble
and chromium III chromate is insoluble. |
I find that hard to believe. Chromate really only exists in neutral to alkaline conditions. In these conditions Ni(+2) would precipitate as
Ni(OH)2 (Ksp = 2.0 10<sup>-15</sup> . .
In more acidic conditions chromate becomes dichromate.
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am going to recommend studying pourbaix diagrams for these kinds of questions.
https://www.materialsproject.org/#apps/pourbaixdiagram/{"chemsys"%3A["Cr"%2C"Ni"]}
(You will need to log in.)
Of course they tell you nothing about kinetics, but at least you can configure your reaction conditions in order to make your target compound stable
and you will also have a clear idea of what other species may be present.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Thanks you all replies folks.
I was thinking right now about the oxalates.. I found some sources saying that the chromium II oxalate to be soluble in water, whereas nickel oxalate
*2H2O is almost insoluble. But I dont know any solubility data regarding chromium oxalates with Cr at higher oxidation states. Also this route can be
problematic if iron is present in the alloy, as it will ppt out along with nickel.
Have anyone data on solubilities of Cr (II, III, etc) oxalates?
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
DraconicAcid
International Hazard
    
Posts: 4403
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Oxalate will from a complex ion with chromium(III).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6359
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I know my link did not work. But it did contain the answer to your question (and a lot more besides.)
Pourbaix diagrams show pH on one axis and oxidative potential on the other. They show what ions and precipitates will be present for any combination
of these two parameters. The entire region is divided into zones so that you can see what is stable under what conditions.
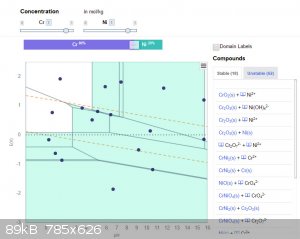 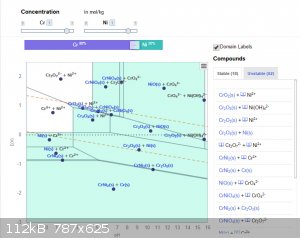
Typically, there are dotted lines shown that indicate stable aqueous conditions. Outside those dotted lines you can potentially oxidise to produce O2
or reduce to produce H2. (It does not always happening depending on what other redox reactions are occurring.)
Anyway, for the Cr/Ni at high concentrations, this is what the diagrams look like...
(The labels do get in the way but when you view it online there is a nice mouse-hover that tells you what is stable in each zone.)
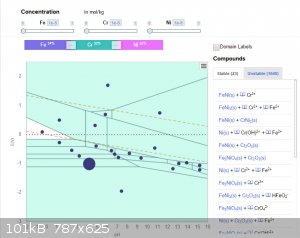
You can also see what happens when Fe is added to the system. Regions shaded blue are where there is an insoluble component. Even at very low
concentrations, inclusion of Fe will result in a lot of precipitate -- which may or may not be helpful in any given application.
[edit]
Adding link that works for generating Pourbaix diagrams.
https://www.materialsproject.org
Again, you will need to log in -- use g+ or fb or whatever.
[Edited on 19-2-2016 by j_sum1]
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
So if I dissolve 80/20 (Ni/Cr) stuff in sulfuric acid/H2O2 would I obtain nickel II and mostly chromium III in solution?
http://www.doiserbia.nb.rs/img/doi/0352-5139/2002/0352-51390...
| Quote: |
Abstract: By combining electrochemical corrosion rate measurements and spectrophotometric analysis of the electrolyte it was shown that at room
temperature chromium dissolves in deaerated 0.1 M Na2SO4 + H2SO4 (pH 1) solution as Cr(II) and Cr(III) ions in he ratio Cr(II) : Cr(III) 7 :
1. This process was stable over 4 h without any detectable change. The total corrosion rate of chromium calculated from the analytical data
is about 12 times higher, than that determined electrochemically by cathodic Tafel line extrapolation to the corrosion potential. This finding was
confirmed by applying the weight-loss method for the determination of the corrosion rate. This enormous difference between these experimentally
determined corrosion rates can be explained by the rather fast, “anomalous” dissolution process proposed by Kolotyrkin and coworkers (chemical
reaction of Cr with H2O molecules) occurring simultaneously with the electrochemical corrosion process. |
EDIT: then adding a soluble oxalate could one separate the Ni(II) from Cr (II and III)?
[Edited on 19-2-2016 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
DraconicAcid
International Hazard
    
Posts: 4403
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
You'll only get Cr(II) if you keep the solution well protected from air. Seriously, separation will be much easier if you oxidize the chromium to
chromate in basic solution and filter out the Ni(OH)2.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW isn't dissolved nickel and hypochlorite a nickel peroxide recipe? The H2O2 process in my hands (on dissolved SS, yes I know about the Fe in that)
had to be boiling hot (with heavy insolubles, mind you), with lots of peroxide turning to oxygen or rather an oxygen-enriched chromate mist.
|
|
|
| Pages:
1
2 |