The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Liquification of oxygen

Oxygen was generated in the 100mL 3 neck flask by addition of diluted 35% peroxide(to around 10-15%) to MnO2. The oxygen was ran through a wash
bottle full of CaCl2, I did not feel like packing a tube. The oxygen was then ran through a dewar condensor filled with LN2. I collected around 5mL
of liquid oxygen.

However, the water in the oxygen generator began to boil, and the 24/40 stopper blew out, so I removed the water in there, closed it up and let fresh
peroxide drip in. While switching it the liquid oxygen I had collected evaporated. I stepped up the peroxide addition to make up my losses, and was
getting an almost steady flow of liquid oxygen out of the condensor, but I was not paying attention to the liquid level in the 100mL flask. It filled
and liquid went up the side tube of the addition funnel, dripped into the fresh peroxide, and blew off every stopper there. oops. By the time I
fixed it the liquid oxygen had evaporated.
In conclusion: I need a bigger gas generating flask, but seeing as I had a difficult time cleaning the glass after the MnO2 in there, I will just get
a cylinder of oxygen soon and try this again. Collecting the liquid oxygen in a small dewar would also be a good idea. I will see if I could borrow
one from someone I know.
EDIT: Yes I know my lab is a mess...
[Edited on 21-5-2006 by rogue chemist]
|
|
|
Mechaton
Harmless

Posts: 22
Registered: 24-3-2006
Member Is Offline
Mood: No Mood
|
|
Now that is cool.
My lab's a mess too.
|
|
|
lahthffire
Harmless

Posts: 29
Registered: 4-7-2005
Member Is Offline
Mood: No Mood
|
|
Cool! I liquified O2 once as well. I filled a dewar with liquid N2, coiled up a piece of metal tubing, submerged the tubing in the liquid N2, and ran
O2 from a cutting torch tank through it. I collected the pale blue liquid O2 in a beaker.
One of my friends was messing around with some burning paraffin wax in a coffee can, and we got the bright idea to pour some O2 onto it. We taped a
styrofoam cup onto the end of a 20 foot long stick, put ~50-100 ml O2 (liq.) into it, then carefully poured it over the burning paraffin wax. We were
expecting a big fireball, but what we got was an intense, loud, explosion! It sounded like a bomb went off! Of course we ran inside and turned off all of the lights after making sure nothing was going to burn down. Of course we ran inside and turned off all of the lights after making sure nothing was going to burn down.
And of course, that was a long time ago; I'm much more mature these days! Well....
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Why not just use dried air? Argon, CO2, and O2 should condense. The cold N2 could be used to precool the dried air.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
You don't need to build any apparatus if you want LOX.
Get some tinfoil (normal household Al foil) and fold it into a conical shape, with a closed tip on the bottom.
Suspend it from a stative with strings.
Test for leaks: fill it with water. No water must drip out anywhere.
Dry it, and fill the thing with liquid nitrogen.
LOX will condense on the outside from the air and drip down at an amazing rate, way faster than if a metal container is used, due to the extremely
efficient heat transfer through the thin Al foil.
Collect the LOX in a test tube cooled from the outside with LN2.
You will see its blue color, and if you drip some on burning wood or paper, huge eruptions of flames will be produced.
A cigarette soaked in LOX will burn fiercely and be consumed in a matter of seconds.
Frost will collect on the outside of the thing after short time and act as an insulator, slowing down LOX condensation.
Just empty the thing, and dry the outside with a heat gun.
The LOX produced this way is of course not very pure, it should better be called "oxygen enriched liquid air".
However, the oxygen content is high enough to allow the blue color to be seen (liquid air is nearly colorless).
The oxygen content can be increased by allowing a portion of the liquid to evaporate by removing the LN2 cooling on the outside of the test tube.
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Where did you get liquid nitrogen?
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
You can get it at any major gas supplier (i.e. praxair, air liquide, linde, matheson trigas etc.). Prices around here are about 60-70 cents a liter,
and sometimes the guys will fill up a 35L dewar for free.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
After reading this last response I called up my local gas supplier. I told him I had a 1-liter dewar. He said he could fill this with LN2 at any
time. I had no idea it was this easy.
This seems like it would be a colder, cleaner, and cheaper method of obtaining a cryogenic bath than using an acetone/dry ice mix. Is this true?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
lahthffire
Harmless

Posts: 29
Registered: 4-7-2005
Member Is Offline
Mood: No Mood
|
|
I was once in need of a cold bath for condensing an amine gas. I usually used dry ice/acetone but was out of dry ice, so I used liquid N2 instead. It
worked well, but perhaps a little too well; the amine froze and plugged up the tube entering the condensation flask. Then the increased pressure on
the generation system caused stoppers to pop out and other unpleasantness!
It's certainly colder. Maybe cleaner, but I don't really think of dry ice/acetone as all that messy. I don't know if it's cheaper or not, I never
payed attention to cost.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
LN2 is much easier and especially cheaper for me to obtain (although it still costs 2,60€ per Liter) than dry ice, the supplier is only ten minutes
away. They even let you borrow a 10L dewar for free.
However, the disadvantage is that you can't liquefy ammonia as nicely as you can with dry ice. It's way too cold for some applications.
It might be an idea to stir LN2 into acetone to cool it down to the required temperature, so that the ammonia doesn't freeze like it will do with
plain LN2. You could even make "icecubes" out of solid acetone, and put these into liquid, precooled acetone to increase the cooling capacity of the
cold bath.
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
this is fricken awsome!!!!!!!!!!!!!!
|
|
|
Pyridinium
Hazard to Others
  
Posts: 258
Registered: 18-5-2005
Location: USA
Member Is Offline
Mood: cupric
|
|
| Quote: | Originally posted by garage chemist
A cigarette soaked in LOX will burn fiercely and be consumed in a matter of seconds. |
... or it might explode outright ....
|
|
|
alancj
Hazard to Self
 
Posts: 76
Registered: 16-6-2006
Member Is Offline
Mood: No Mood
|
|
What I have always wondered is how hard it would be to build a DIY refrigeration system to generate LOX. Something like this.
[Edited on 29-5-2007 by alancj]
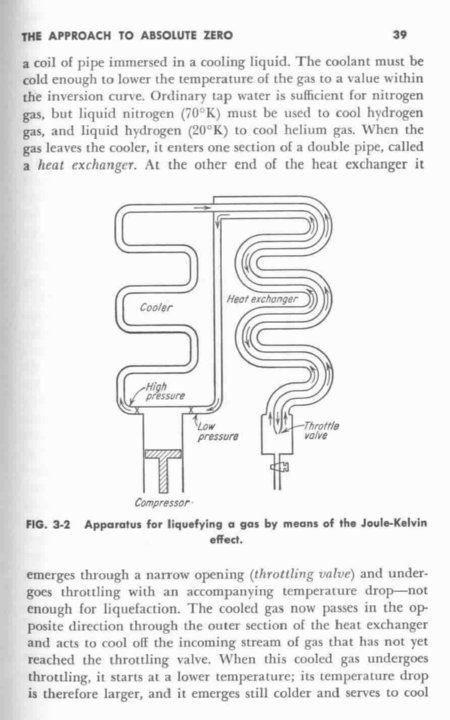
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Ok, i am over my previous excesive excitment as with my last post.
I called all the gas suppliers in the phone book. All except for one said no way. The one person who said it would be possible for an individual to
purchase said I would need a dewar flask. The cheepest one: 595 USD!!!!!I asked him about thermos bottles and he was steadfast that they would not
work. It seems to be the only gas supplier around. Any ideas?
Thanks
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
you can find cheaper one on eBay or labX, or check that website for new smaller one: http://www.cryodewars.com/index.php?cPath=1_2
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
<<< The cheepest one: 595 USD!!!! >>>
That's b/c you need a pretty big one.
use of a thermos bottle will not git yer L2 home B4 it evaporates............
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Its not true, a thermos bottle will hold LN2 quite well. Of course not as long as a dewar, but at least 24 hours for a two liter thermos.
A thermos would be useable for carrying LN2 home and using it immediately.
Remember to drill a small hole into the stopper.
Dont buy new dewar flasks, they all cost a fortune as you have seen.
Look on ebay for used ones.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Would it be worth it to make a dewar vessle yourself? It would require glass bottles which could be silvered using tollens reagent and glucose,
styrofoam could provide a lot of additional insulation, the hardest part would probalby be find bottles that fit into eachother. Perhaps using tall
beakers might work of you make a stopper of really thick (silvered?) styrofoam. Polyurethane foam might be evan more usefull in this case you could
just spray it between the two bottles.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
Drone
Harmless

Posts: 12
Registered: 20-11-2005
Location: UK
Member Is Offline
Mood: No Mood
|
|
The trouble with making a dewar oneself are twofold:
Insulation quality:
LN2 has a fairly low specific heat capacity. The insulation does not have to be that far off perfect to sustain quite rapid boil-off.
Materials:
LN2 has a boiling point below that of oxygen. Thus if a porous foam is used as insulation, LOX condenses and bad things happen. Quite a few materials
are shock sensitive when soaked in LOX.
Yet another problem is that common polymers tend to be quite fragile at low temps.
Saying that though. It certainly is possible to store LN2 for short periods of time in a vessel insulated with a suitable closed cell foam. You might
even be able to purchase these relativly cheaply, as these things quite often turn up in undergrad. physics labs to store LN2 for a few hours.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I recall seeing perlite is suitable for a suprising insulation capacity. Vermiculite may also be good.
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Using foam as the primary insulation has one other potential problem. Consider what is making the voids in the foam - what gas is filling the cells?
Simply cooling the foam down a lot is going to reduce the pressure in the cells, if they get cold enough the gas will condense to a liquid. Foaming
agents that I know of are low boiling alkanes, fluorocarbons, and CO2.
An outer wrapper of foam can help cut down boil-off a bit, and provides mechanical protection.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I think it would be nice, if you were too poor to buy your own DEWAR, to design a fake one so your supplier wouldn't know it wasn't actually a DEWAR.
Surely it wouldn't be too hard to make something LOOK like a DEWAR.
I wouldn't recommend it. Just speculation.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|