xxxxx
Hazard to Others
  
Posts: 116
Registered: 21-5-2004
Member Is Offline
Mood: No Mood
|
|
abnormal nitration of styrene
i once read that supposedly when nitric acid is added to styrene the nitro group adds to the beta carbon without affecting the double bond to form
b-nitro styrene and one molecule of water. when i tried this there was evolution of heat as the reaction is strongly exothermic, the reaction mixture
turned brown and there was a strong odor resembling cinnamon. could anyone speculate whether b-nitro styrene was in fact the product?
|
|
|
joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
cinnamon odour is a feature of beta-substituted ethylbenzenes, so you might indeed have made the b-nitrostyrene.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The nitration of styrene yields a terrible mixture of products. Depending on the conditions there some beta-nitrostyrene can form but note as the only
product.
See:
Nitrations with Acetyl Nitrate. II. Nitration of Styrenes and Stilbenes[url=http://dx.doi.org/10.1021/ja01499a029]
Nitrations with Acetyl Nitrate. I. The Nature of the Nitrating Agent and the Mechanism of Reaction with Simple Alkenes[/url]
(Let me know if you can't access to ACS jurnals so I can upload them somewhere)
There is a case of direct nitration of styrene with HNO3 formed in situ from KNO3 and polyphosphoric acid. The yield of the beta-nitrostyrene is 37%
though. If you understand Russian see:
Nitrovanie olefinov nitratami natriya, kaliya i ammoniya v polifosfornoj kislotezh. Organ. Himii, 26 (1990) 680-681.
Of course there are numerous other elegant methods for the transformation of styrenes to beta-nitrostyrenes: the pseudonitrosite
formation/decomposition, I2/NaNO2 or I2/AgNO2, nitration with C(NO2)4, nitration with NO in presence of basic Al2O3, etc.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
More info please.
Physical properties of other products of this reaction etc...
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Demanding information on a 12 year old thread. Nice!
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
Why not when it's very interesting 
I though to distill/steam distill to obtain b nitro,but i think it would be messy :/
Some better ideas are welcome,for sure.
It's still faster to do this method than others,and more available...
But nothing is perfect...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
If your target is 1-nitro-2-phenyl-ethene...the best yielding option is to go from benzaldehyde and nitromethane.
Alternatively...you may start from styrene...add bromine (without heat, light or catalyst (thus no AlBr3) to get dibromo-styrene...this may react with
AgNO2 in a good solvent (DMF/DMSO)...the easiest accessible halide is the one on position 1 (the more external)...so you will get
1-nitro-2-bromo-2-phenyl-ethane; the later will be very happy to dehydrohalogenate with a base to get an harmonic double bond in resonance with the
aromatic ring.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
Yes i know for condensation reaction,but i don't have nitroalkanes...
This second reaction that you mentioned is interesting.
But i do not have that,out of halogens i have chlorine,iodine,of nitites,sodium nitrite,so can something be modified,i hope so.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
In principle chlorine would lead to the desired 1,2-dichloro-2-phenyl-ethane...this may be use straight although it will be less reactive with NaNO2.
To increase the reactivity of the halide you may exchange the 1-chloro by a 1-iodo via Finkelstein reaction (aceton saturated with NaI...the NaCl from
the halide exchange is less soluble into aceton and precipitates out of solution.
Then the iodo compound will be more reactive towards NaNO2 or AgNO2...
I don't know if the 2-chloro will be exchanged too...but even if it happens, it is not a real problem because similar case as the
alfa-beta-dibromostyrene...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Would this work with propenyl/allylbenzenes also?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The addition of the halogen yes...
The substitution by NO2 not for the first but wel for the second...
The elimination of the halogen to reform the double linkage should work and even eventually a triple linkage with dihalo-propyl-benzene...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Why couldn't you use iodine instead of bromine or chlorine? It adds to double bonds reliably enough that it's a standard metric for describing
triglycerides. Or is iodine too specific in its activity?
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by Melgar  | | Why couldn't you use iodine instead of bromine or chlorine? It adds to double bonds reliably enough that it's a standard metric for describing
triglycerides. Or is iodine too specific in its activity? |
Not only can iodine be used instead, but the beta-nitrostyrene can even be obtained in a single reaction if the alkene is treated with I2
and NaNO2 in water and ethyl acetate (ether may also be used) while in the presence of a diol like ethylene or propylene glycol. Unlike the
other methods, the I2 + NaNO2 route does not proceed through a 1,2-dihalide intermediate. Instead, the nitrite salt reacts with
the iodine to give nitryl iodide, which then undergoes homolytic fission to give atomic iodine and nitrogen dioxide. The iodine radicals can then
react with other nitryl iodide molecules to give back I2 and produce more nitrogen dioxide. The nitrogen dioxide radicals then attack the
alkene bond to form a nitroalkyl radical (stabilized by the aromatic ring in this case), which gets rapidly quenched by the I2 to give an
iodonitro intermediate and another iodine radical:
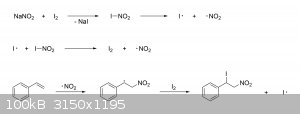
The iodo group is then eliminated through the usual dehydrohalogenation mechanism to give the nitroalkene. Anyway, below are a couple of links that
may be useful for anyone interested in trying this. It's probably worth mentioning that an inert atmosphere significantly improves yields (or so the
authors claim).
A Practical Preparation of Conjugated Nitroalkenes
Nitration of Propenylbenzenes with Nitryl Iodide
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Nice finds................ I just went looking for them.
Tsathoggua1 check 'em out............................
[EDIT] - There is also this here...................
http://www.sciencemadness.org/talk/viewthread.php?tid=5255
/CJ
[Edited on 26-2-2017 by Corrosive Joeseph]
[Edited on 26-2-2017 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|