Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Conversion of Phthalic Acid to Phthalic Anhydride
August 10, 2013
by Magpie
A. Introduction
“Phthalic acid can be converted to phthalic anhydride by heating the acid above 180°C. Unlike most such conversions this happens with ease due to
the formation of a 5-membered ring.” (ref 1)
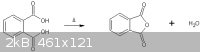
The following procedure will yield ~19g of phthalic anhydride using a charge of 25g of phthalic acid. This provides a %yield of ~98%.
B. Chemicals
25g of phthalic acid
~ 500 mL of silicone oil (note 1)
C. Equipment
1. magnetic stirrer-hot plate
2. magnetic stir-bar
3. 1- liter stainless steel bowl
4. thermometer (≥210°C)
5. PID controller (optional)
6. 7.5cm ID iron ring w/clamp
7. ring stand
8. 250mL beaker
9. stainless steel spatula
10. evaporating dish
11. dessicator
12. mortar & pestle
13. fume hood (note 2)
D. Procedure
1. Place 25g of phthalic acid in the 250mL beaker.
2. Place the stirrer-hot plate on the ringstand.
3. Add about 500mL of silicone oil to the stainless steel bowl.
4. Place the oil bath on the stirrer-hot plate and add the stir-bar.
5. Attach the iron ring to the ringstand.
6. Insert the 250-mL beaker with phthalic acid in the iron ring. The beaker should rest securely on its lip.
7. Bring the beaker down into the oil such that the oil level is above that of the phthalic acid.
8. Turn on the magnetic stirrer such that it stirs the oil thereby promoting good heat transfer and even heating.
9. Support the thermometer such that it indicates the oil temperature.
10. Turn on the fume hood fan.
11. Heat the oil slowly and steadily until it reaches a temperature of 195°C. Use the PID controller to maintain the bath temperature if available.
12. As the phthalic acid begins to heat up and liquefy (mp = 131°C) water vapor will evolve. Some of the formed phthalic anhydride will vaporize
forming filamentous crystals (wool) around the lip of the beaker. Gather the wool as it forms using the ss spatula and drop it back into the melt.
See photo below:

13. The melt should be gently boiling and off-gassing. Continue this until the milky melt turns clear.
14. Turn off the heat to the oil bath. When the bath temperature reaches 175°C pour the melt into an evaporating dish. Place the dish in the
dessicator.
15. When the phthalic anhydride has cooled and solidified grind it to a powder using a mortar & pestle.
E. Results
1. Weigh the powder; it should weigh ~ 19g. On his last run the author obtained 19.4g for a %yield = 98.6%.
2. Determine the melting point. The author obtained a mp = 131.5°- 132.0°C. (Wikipedia mp = 131°C)
F. Discussion
This procedure was developed out of the author’s frustrated attempts to obtain phthalic anhydride from three vendors, ie, Elemental Scientific,
Antec, and Chemsavers. In each case the author was sent what appeared to be phthalic acid as determined by melting point (mp = 191°C, sealed tube).
See ref 2.
The experimental work for this procedure was performed in 2010 as indicated in ref 2.
G. Notes
1. Any suitable oil capable of reaching at least 200°C safely may be used. Silicone oil is preferred due to its relative inflammability and low
odor.
2. In lieu of a fume hood this procedure can be conducted outside.
H. References
1. Wikipedia (2010) entry for “phthalic anhydride”
2. Science Madness Forum posts for “Phthalic Anhydride”, 2010: http://www.sciencemadness.org/talk/viewthread.php?tid=10156
[Edited on 11-8-2013 by Magpie]
[Edited on 11-8-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Is it still the same story r.e. phthalic acid being sold by suppliers in place of the anhydride? Has anyone purchased some recently?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I've been running reax above 200 with dibutyl phthalate. It's cheaper than silicone oil. I don't use oil often but for this I would .. or sand. It
is ironic that this should be one of the few acid to anhydride conversions that happens with heat. I remember writing the little delta underneath the
arrows for *every* acid -> anhydride.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is a warning to those wishing to use this procedure for converting phthalic acid (PA) to phthalic anhydride (PAN). After
finding that some of my supposedly converted PAN was actually PA due to a failed synthesis I now recommend that you collect the PAN as wool to be sure
that you have PAN. Brewster, p. 119 (forum library) gives an easy procedure for discriminating between PA and PAN. If you have any questions, let me
know.
[Edited on 12-12-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
It's a good practice, because only the pthalic anhydride can be sublimed well, and both phthalic anhydride and phthalimide heavily fume and sublime
all over your device, so having a lot of sublimed material is a good sign that reaction is sucessfull.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by manimal  | | Is it still the same story r.e. phthalic acid being sold by suppliers in place of the anhydride? Has anyone purchased some recently?
|
I recently purchased 2.5 kg from Antec, a supplier in my city, and it is indeed the anhydride. I've sold it to several members and I have a bit more
if you're interested.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
When my first synthesis of 2-octylhydrogenphthalate failed I checked the mp of the PAN I had made using my procedure for converting PA to PAN. The mp
was good at 131°C. Apparently there was enough PAN to lower the mp of the PA which is 207°C. This thru me off. I did do the chloroform
dissolution test, however, and it clearly showed that most of it was PA. So I sublimed the wool to be sure I had PAN.
I ordered PAN from 3 suppliers: Antec, Elemental Scientific, and Chemsavers. All 3 sent me PA. I determined this from their mp's. So I recommend
being skeptical. Check your PAN with the chloroform test. If in doubt, sublime the wool.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So ... if you buy pthalic anhydride the recommendation is to sublime it to ensure that actually is the reagent you expected to get ?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Not quite. I say be a little skeptical and test it with chloroform. PAN dissolves in chloroform; PA does not.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Excellent practical and substantiated advice.
Thankyou.
|
|
|