thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
Digestions with nitric acid
I'm doing some 16th century experiments with cobbled ware.
First issue: I need a luting material/putty that stays soft enough to remove but firm enough to seal, that won't contaminate the experiment, and
stands up to boiling nitric acid. Henley's manual mentioned asbestos and portland cement but not a carrier, and even if asbestos were still available,
this is not meant to be a permanent seal!
The vessels are 1 gal jars joined at their mouths by a ceramic collar, the lute will seal the collar to the jars.
The idea is to boil the acid in the lower container and let it cool in the upper one.
Is this a safe set-up? I'd like to add some kind of pressure regulator/expander, to observe and mitigate pressure increase, like a balloon or bellows
but can't find anything off the shelf that can stand up to hot N' acid.
Again, this is not a distillation, but a digestion experiment, that lasts about 8 hours.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4348
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Wait- you want to seal two jars together, with nitric acid in them, and then boil the acid?
NOT a safe idea.
What you want to do is reflux the acid- have a container of boiling acid with a condenser attached to it, so that the acid vapours condense into a
liquid and dribble back into the jar. This is not sealed, so the contents do not get pressurized. You would probably want to cap it with a
bubbler/trap to capture any nitric acid vapours that try to get away.
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
"Wait- you want to seal two jars together, with nitric acid in them, and then boil the acid?
NOT a safe idea.
What you want to do is reflux the acid- have a container of boiling acid with a condenser attached to it, so that the acid vapours condense into a
liquid and dribble back into the jar. This is not sealed, so the contents do not get pressurized. You would probably want to cap it with a
bubbler/trap to capture any nitric acid vapours that try to get away."
-------------------------------------------------------------------------------------------------------
In a worst-case scenario I would expect the lute to give way. Can you recommend a luting material? Also could you direct me to a diagram illustrating
how such a set-up works? I've been having difficulty finding clear illustrations on google. Also to be honest I don't get how it works in principle,
because any dribbling ingress into the jar would have pressure pushing it back into the condensor. What you're describing still sounds like a closed
system. If it's not a closed system then vapors do get out.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4348
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
A bubbler would look like this:
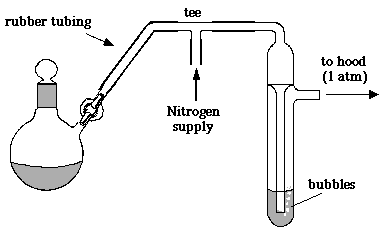
Pressure can't build up, but nitric acid vapours dissolve in the water (or better, a solution of sodium carbonate).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Generally not a good idea! You're not one of those vegan homeopathy alchemy 'healing power' of crystals weirdo's, are you?
If you're trying to replicate a 16th century <em><a href="http://en.wikipedia.org/wiki/Aqua_fortis" target="_blank">aqua
fortis</a></em> <img src="../scipics/_wiki.png" /> digestion, is there any particular reason you can't use modern glassware?
I had to look this up:
<a href="http://en.wikipedia.org/wiki/Lute_(material)" target="_blank">Lute</a> <img src="../scipics/_wiki.png" />
[Edited on 7/9/13 by bfesser]
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
Draconic: Thank you for the diagram. (I don't know what the nitrogen port is for, I'm not adding nitrogen.) But I gather that the bubbler reduces
chance of accident by releasing pressure at water surface tension. The problem with that is as the flask cools down it tends to suck water
IN! (I inadvertantly broke a few flasks with inrush of distillates.) So I gather that I'm to remove the bubbler before the flask has had much
chance to cool down?
bfesser: Well maybe I AM!  but more-or-less quit the vegan experiment years ago.
Anyway this is about "beginnings," right? Just because something is "new" doesn't make it better. I have a lot of respect for what the alchemists
accomplished without modern glassware. but more-or-less quit the vegan experiment years ago.
Anyway this is about "beginnings," right? Just because something is "new" doesn't make it better. I have a lot of respect for what the alchemists
accomplished without modern glassware.
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
unnecessary quoting]
[Edited on 7/9/13 by bfesser]
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
...and I still need a nitric-acid resistant putty that doesn't cost me both arms & legs.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4348
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by thrival  |
Draconic: Thank you for the diagram. (I don't know what the nitrogen port is for, I'm not adding nitrogen.) But I gather that the bubbler reduces
chance of accident by releasing pressure at water surface tension. The problem with that is as the flask cools down it tends to suck water
IN! (I inadvertantly broke a few flasks with inrush of distillates.) So I gather that I'm to remove the bubbler before the flask has had much
chance to cool down? |
The nitrogen inlet simply came with the diagram- the first useful one I found on google. As the flask cools down, it will suck in air through the
bubbler- you don't have so much liquid in it that it will not let air in at all.
For a nitric acid-proof sealant, you *might* be able to use silicone.
[Edited on 15-2-2013 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
teflon tape is cheap at harbor freight and is soft and makes a good seal,it is not 16th century but who will know?
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
Draconic:
&
cyanureeves:
I just found a PTFE gasket sealer called "Chesterton 3500 Valvelon" it comes in a roll, you pull off what you need and smush it in like a putty,
haven't priced it yet, if too expensive will probably just go with PTFE paste and cut it with some clay powder. It's the carrier oil I'm concerned
about. Dupont makes a PTFE based oil & putty called Krytox that is way pricey. PTFE tape doesn't have enough body to seal gap between wide mouth
jar openings and ceramic collars, that's why I need a putty, but thanks. Also I believe the old alchemist digestion apparatus were closed systems, the
trick is having enough room for the vapors to expand. I'm doing this to save money and prove it can be done without expensive labware, not to be
"quaint."
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
...also the bubbler concerns me because any gases that leave by that route during expansion will want to come back in as the flask cools (creating a
vaccuum) that will want to pull water in, which would ruin the experiment, or the jars could implode.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
Well, I can't imagine that the jars would implode. Bubblers just don't work that way. What you could do, if you were very worried about suckback, is
to set up a suckback trap: a flask between your reaction vessel and the bubbler so that gasses go through it and if suckback occurs, the liquid would
fall into the flask.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
As for respecting the alchemists. A lot of them died as a direct result of their experiments. It's mostly the ones who lived long enough to report
their results that you can now read about—some of whom were just plain lucky, some of whom had sacrificed 'assistants' rather than
themselves. I hope you don't value your physical well being.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Please DO NOT USE A HOMEMADE SEALED VESSELwith nitric acid- too dangerous PERIOD. Set up for standard reflux.I think using an exit bubbler is probably
overkill.Use chilled water or your condenser (homemade or otherwise) and the escape is negligible.Just so long as the vessel is open to the
atmosphere.
A sudden explosive spray of nitric acid is not to be fucked around with!
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i am with bfesser on this one because i too read about the life expectancy of the old masters but was convince that i had superior material than they
did.i used to wedge oil lamp tubes into glass coffeee pots and use rolls and rolls of teflon as gaskets and even though the red nitric fumes still
pushed the glass apart,nitric acid still condenses enough to collect inside in a puddle.i have not read anything about nitric causing loss of sight
but i swear my sight was alot better before i breathed all the fumes. its fun to walk in the steps of the old arab alchemists but the outcome might
not always be worth it.
|
|
|
thrival
Harmless

Posts: 23
Registered: 14-2-2013
Member Is Offline
Mood: No Mood
|
|
I really do appreciate everyone's concern about safety.
Again, this isn't a distillation and there is no distillation tube.
The digestion vessel I read about is a jar or wide mouth flask with a capacious cooling head, that fits inside, and a soft lute seal. A one gallon
reaction vessel would contain a quart or less of acid. The vapors collect in the upper head, condense and run back down. An active boil isn't
required. The lower vessel is coated with a ceramic concrete like material that contains leaks if the vessel were to break, and can withstand high
heat, the latter which I don't expect to be using. I figure the guys who lived, did know what they were doing. Of course I can add a pin-hole in the
lute or a narrow bubbler tube, thanks for the suggestion.
Have found fluorinated oils and greases are too damn expensive!
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
there was a video on youtube with no sound of an arab making nitric acid simillar to what you are doing except he used stainless steel. a 3gal.
stainless tub was used with a conical lid, the cone point was upside down in the tub and filled with ice.inside the tub wall was welded a spiral track
that led to a spout that dripped out nitric acid .your type of set up has been discussed here before and even drawn in diagrams. i think i read that
stainless steel passivates and stops reacting with nitric and was possible to use as a vessels.again not 16th century and have fun!
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by thrival  |
Draconic: Thank you for the diagram. (I don't know what the nitrogen port is for, I'm not adding nitrogen.) But I gather that the bubbler reduces
chance of accident by releasing pressure at water surface tension. The problem with that is as the flask cools down it tends to suck water
IN! (I inadvertantly broke a few flasks with inrush of distillates.) So I gather that I'm to remove the bubbler before the flask has had much
chance to cool down? |
The nitrogen inlet simply came with the diagram- the first useful one I found on google. As the flask cools down, it will suck in air through the
bubbler- you don't have so much liquid in it that it will not let air in at all.
For a nitric acid-proof sealant, you *might* be able to use silicone.
[Edited on 15-2-2013 by DraconicAcid] |
I have personally made a dodgy homemade condenser (now retired) that used silicone to seal the garden hose to the fluorescent light globe condenser. I
then used this to make nitric acid, and as such can confirm that silicone seals in both nitric acid vapors and NO2 fairly well, although it turns
yellow fairly rapidly and after long exposure becomes quite brittle.
Also @thrival this is a science forum, do please use SI units (you know, those sensible ones. liters, meters, pascals etc)
|
|
|