Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on attempt to make Nickel Iodide by double displacement
I was keen to try to make NiI2 after reading that it has a blue-green hydrate. I decided to go for a double displacement reaction with lead iodide.
NiSO4+PbI2=NiI2+PbSO4
I had nickel carbonate from a previous experiment, and that was reacted with sulphuric acid to make nickel sulphate in solution. It should have been
5g NiSO4 in solution and this was to be reacted with 15g PbI2. From having used lead iodide before to make other iodides via double displacement I
knew the reaction had to be done in a large volume of water due to the low solubility of lead iodide and that it would take time.
The NiSO4 solution and PbI2 was added to approx. 400ml water and stirred for 36 hours. See photo. The yellow color faded a bit but not as much as I
expected. Also at that time my hotplate failed so I would not keep the solution warm to get more lead iodide to dissolve.
After 36 hours I stopped stirring. A mix of unused lead iodide and lead sulphate settled and the green solution was boiled down. See photo.
I stopped boiling it down at approximately 30ml volume and transferred it into a crucible. There was a very fine black residue in the still-hot beaker
that instantaneously disappeared when it was sprayed with cold water. I think it was a bit of anhydrous nickel iodide; according to Wikipedia the
hexahydrate loses water at 43C.
The crucible went into the desiccator for 24 hours. High vacuum and over NaOH. Unfortunately it seemed to not only dry the compound but also dehydrate
it. What was left in the crucible was a dark brown-black hard layer. This was scratched out and transferred to a vial, 6g was recovered. See photo.
The bits left in the crucible was seen to be turning green after some 10 minutes. A bit of water was added, it dissolved easily to a dirty green
solution. A few drops of lead nitrate solution was added, and the expected yellow ppt of lead iodide observed.
So while I seemed to have gotten nickel iodide NiI2, it was not the green-blue hydrate I was hoping for.
As an aside to the main experiment, I then wanted to recover the unused lead iodide. But this was mixed with lead sulphate. By this point I had a hot
plate again, so the beaker was filled with some 600ml water, boiled to dissolve some lead iodide, filtered hot, and the solution then cooled to allow
the lead iodide to ppt out before decanting off the clear solution and repeating the process twice more. This of course had the bonus that I could sit
and look at 'golden rain' as the PbI2 condensed out of the cooling solution. This is always beautiful to watch! See photo. Some 3g lead iodide was
recovered.
   
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
I acquired some nickel powder a while back and decided to see if it is easier to make nickel iodide starting from the metal powder and iodine.
And....yes it is.
- 6g nickel powder and 30g water in a small beaker, with stir bar.
- Add 3g iodine crystals:

- Heat till I2 fumes are coming off and then switch off the heat. This gave a dark solution from the iodine dissolved in water:

- The colour of the solution turned to green within minutes, with all the iodine consumed:

- Add iodine in 2 gram portions, still until green, repeat. At this point the reaction kept the solution quite hot, but no so hot to release iodine
fumes.
- Continue until a total of 15 gram iodine was added. Stop for night.
Next day:
- Filter twice to remove the unreacted nickel powder, this was the final filtrate:

- On steam bark quickly dried to a black crystal layer, but still green liquid underneath:

- Left out overnight, next day it was all wet again:

- Place in desiccator for 72 hours to see if the green hydrate could be extracted. But it did not dry much, and the part that dried was again the
black anhydrous salt.
- Place in sandbath on low heat:

- After an hour or so there was only dry hard black crystals left. There was no sign of decomposition; no smell and no iodine fumes.
- 15.2g anhydrous NiI2 recovered, a yield of around 82% based on the 15g iodine.

Tests on residues:
- I added water to the evaporating dish, after 24 hours the crystals had dissolved into a green solution:

- Added lead nitrate to the green solution, resulting in a ppt of yellow lead iodide:

Nickel iodide is thus quite easy to make direct from the elements, in water. Any suggestions of a use for NiI2?
[Edited on 11-8-2021 by Lion850]
[Edited on 11-8-2021 by Lion850]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
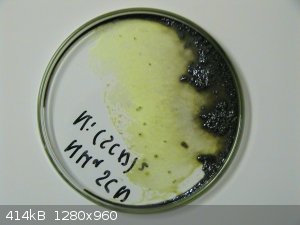
Interesting that you can make it directly from the elements, I had expected nickel to be passivated by its oxide layer and no reaction to take place.
Your product is dark grey-black? Interestingly, I once tried to prepare nickel thiocyanate from wet nickel hydroxide and ammonium thiocyanate.
Initially, the solution was nickel-green, but on evaporation, it became more yellow and ultimately I obtained a dark brown, almost black compound with
a metallic luster.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Bezaleel I found some supplier photos of nickel iodide online and it looks very similar.
From what I have seen so far manganese, nickel, and iron in powder form will all react with iodine in water, sometimes just needs a little bit of heat
to get going. I do have lead powder; I may try lead sometime just to see if anything happens.
I would like to isolate dry the green hydrated nickel iodide salt, but it loses its water under 50c so the only way with what I have may be to make a
large amount and rely on change in solubility as the solution cools. It will take a lot of experimenting.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Lead powder may not work, since PbI2 may form a protective layer, but you only know after you've tried. (Like no-one believed Al-foil will dissolved
in household ammonia solution - but it does, though slowly.)
You said nickel iodide loses water under 50C. What do you mean with that? Do NiI2.nH2O crystals weather? For hygroscopic solutions - I usually force
them to give crystals by extracting the water under vacuum in a desiccator over NaOH.
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
@lion850 Nice work! I made the gold rain experiment at highschool chemistry and they let us take it home! Ah those were the days! I have
entertaining the thought on using lead bromide and lead iodide in double displacement reactions. This post confirms my thoughts. To all who are
reading, if lead salts ain’t your bag, you can always try the alcohol/potassium sulfate method if you have a potassium salt with high solubility.
Check out this link on how to prepare Ammonium Iodide.
[Edited on 9/27/2024 by chloric1]
Fellow molecular manipulator
|
|
|