SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Problems with the synthesis of formaldehyde by pyrolysis of metal formates
Hi,
I have been attempting to make a solution of formaldehyde, by passing the gases produced by the pyrolysis of various metal formates into water.
My attempts have been almost completely unsuccessful, although its proving quite a fascinating mystery.
Requiring a small quantity of formaldehyde (mainly for Eschweiler-Clarke methylations), I figured that the pyrolysis of calcium formate would be the
most straight forward method, although I could find no practical write-ups until I found this one:
formic acid to formaldehyde by BASF
These ketonic decarboxylation reactions have fascinated me for quite a while, due to the easy-to-obtain nature of the reagents, but in practice, the
reactions seem less than ideal due to low yields and messy side products.
Although, the formaldehyde reaction must be the cleanest one, as there isn't really much opportunity for side-products that would be hard to separate.
The general reaction is :
(R1-COO)2Ca + (R2-COO)Ca > R1-CO-R2 + CaCO3
Anyway, I made some calcium formate by neutralizing 54% formic acid (descaler) with gardening lime, the solution was filtered and dried on a water
bath, yielding nice white crystals of calcium formate.
I loaded about 30g of the salt into a metal retort (an empty aerosol can), and fitted a glass 3-way adapter, sealed with PTFE tape, to the top.
The 3-way adapter was stoppered at the top, and a vacuum take-off adapter with flask was attached to the side arm of the 3-way adapter, the vacuum
adapter was connected to a tube which was immersed in a graduated cylinder of water.
The vacuum adapter and flask were there to catch any water that may have been sucked back through the tube.
Heating the metal can strongly with a Bunsen burner, some white vapours began to appear in the glassware, obviously with bubbling in the water.
I had weighed the water beforehand, expecting an increase in weight of around 3g, assuming a reasonable yield.
The heating was continued for around 20 minutes, until the rate of bubbling had almost stopped.
The water, upon weighing after the reaction, had increased in weight by no more than 100mg, which would correspond to only a 2% yield!
In spite of this, there was a smell of formaldehyde in the air.
(I don't actually know what formaldehyde should smell like so I'm just guessing, but it seemed right)
Also, there were a few droplets of oily brown liquid condensed on the glass.
Upon cooling, I emptied out the metal can, and found a coarse black powder, which flowed freely from the can.
Adding the powder to acid showed effervescence, completely dissolving except for black bits floating on top of the water.
Thus I would guess that the solid is mostly calcium carbonate, as expected, but the black stuff is carbon.
I heated some of the powder to red heat, and the blackness slowly faded once a red-orange heat was attained, leaving beige calcium carbonate behind;
this further indicates the black stuff is carbon, as it is burning at these high temperatures.
Since this first unsuccessful attempt, I have recreated the reaction many times; Under vacuum, reduced pressure, inert atmosphere (methane), using a
carrier gas (also methane), I have tried mixing the calcium formate with an inert bulking agent (NaCl), I have tried heating it slowly and carefully,
none of these made any difference at all.
I read that "Solubility decreases with increasing temperature and at room temperature polymerization and volatilization occur, leaving only a small
amount of dissolved gas." (here), so, just in case the temperature of the water through which the gas was being bubbled was the problem, I repeated the reaction, but with
ice water, in an ice-bath...
No difference.
So I eventually came to the conclusion that the only reasonable answer is that the high temperatures required in pyrolysis of the calcium formate were
enough to decompose the formaldehyde into its constituent elements, thus forming elemental carbon, this was the only thing I could come up with, but
it still doesn't explain how BASF got it to work so well.
Having tried everything I could think of, I went about trying to find alternative metal formates that would decompose at a lower temperature; Zinc
looked like a good option, so I prepared some (zinc metal from some zinc-carbon batteries was dissolved in HCl, then NaOH added to precipitate the
ZnO, followed by dissolving in formic acid, etc).
At first, the zinc formate looked more promising, as it also evolved a gas that smelled of formaldehyde, but the reaction occured at a lower
temperature, and the substance left behind was white, not black!
The pyrolysis reaction of zinc formate is different from the calcium one:
(HCOO)2Zn > HCHO + CO2 + ZnO
However, attempts to dissolve the gases in water were unsuccessful, with no weight gain measured.
I tried lots of variants of this reaction too, with no success;
Having spent weeks at it, I was almost giving up, when I found this thread where I read "according to Goldschmidt tin formate decomposes almost quantitatively to formaldehyde."
So, I went about looking for the Goldschmidt paper, which has been quite frustrating.
I have found many references to it, in the usual format, but I cannot find the paper, the reference is:
"Goldschmidt, Chem. Ztg. 1907, 48, 608"
It just doesn't seem to be available, but I did find this paper, which gives some useful information about tin formate, but unfortunately no info about the yield of formaldehyde.
I made some tin (II) formate by the method given in the paper above, and ended up with some nice off-white crystals.
The usual pyrolysis setup was used, and so I tried it with the tin salt.
The reaction begins at a much lower temperature, and formaldehyde is definitely produced, as the smell is quite apparent, the residue that remains is
tin (II) oxide, but alas, still no success in dissolving it
And that's basically where I am now, I even boiled down the solution just to make sure that I wasn't making some weighing error, but when I boiled it
down, there was only a tiny trace of a white solid, if it is paraformaldehyde, its only a few milligrams.
Does anyone have any ideas where I might be going wrong?
Would really appreciate any suggestions, its been quite a tricky problem.
P.S. this is (almost) my first post. Glad to finally post something here after spending so much time browsing. Haha
Thanks in advance
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I don’t know German to translate, sorry.
Have fun:
Attachment: Chemiker Zeitung 1907 v31 p608.pdf (1017kB)
This file has been downloaded 412 times
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Could it be that the can is screwing things up?
Seems to me that it reacts with all sort of metal catalysts when hot enough.
Have you tried it in glass?
It's only supposed to take 300 degrees C.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If this is to be believed then the calcium salt is about the worst option
A. Górski, A. D. Kraśnicka. Influence of the cation on the formation of free hydrogen and formaldehyde in the thermal decomposition of formates.
Journal of Thermal Analysis 1987, 32 (5) , 1345-1354. DOI: 10.1007/BF01913334
Fig 1 suggests that zinc formate would be a better choice. (Though their addition of borohydride to the mixture complicates interpretation)
Essentially, if you get carbonate as a product, then that implies that the carbon from the formate was not converted to formaldehyde.
|
|
|
kmno4
International Hazard
    
Posts: 1496
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
By the same authors:
Origin of organic gaseous products formed in the thermal decomposition of formates. Journal of Thermal Analysis 32, 1243–1251 (1987)
(DOI:10.1007/BF01905178)
....and from there:
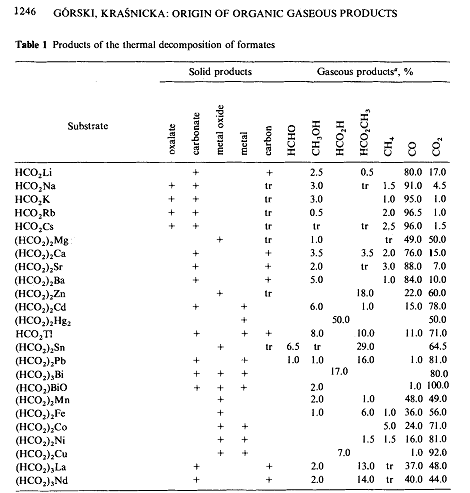
(heating performed under nitrogen)
Слава Україні !
Героям слава !
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you just want formaldehyde, why not buy trioxane or paraformaldehyde?
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Wow thanks! I was just about losing my mind after 10h of searching 
It seems to translate okay with Google, it seems to suggest that the formate should be prepared in vacuum;
The method for preparation I used was refluxing the tin oxide with a large excess of formic acid (10x the stoichometric amount as specified in the
paper), filter, then cool.
The tin formate crystallises out on cooling, and it seems to be quite pure.
Although this is probably the best way to make the pure compound, its not exactly ideal or high yielding, because some of the salt remains dissolved
in the acid, and since the acid is the solvent, it has to remain concentrated, and in the right quantity (too much acid and the salt won't crystallize
out on cooling)
So I tried a different method; dissolve in excess of acid, hot filter, then vacuum distil the solution, recovering the acid, and leaving all the salt
behind in the boiling flask.
This method seems to work, and gives a higher yield, but anyway, that's a side note I guess.
I'll try to make some sense of this paper today
Quote: Originally posted by SWIM  | Could it be that the can is screwing things up?
Seems to me that it reacts with all sort of metal catalysts when hot enough.
Have you tried it in glass?
It's only supposed to take 300 degrees C. |
That's an interesting idea, I did the calcium formate reaction in the metal can due to the temperatures involved, but I have since tried it on a
smaller scale in a test tube with no apparent difference, and I tried the tin formate in a glass flask.
300C is quite high in terms of decomposing formaldehyde though, its supposed to begin decomposing at 150C.
But yeah, glass can survive 300C, I mainly used metal because I was directly heating it with a Bunsen burner.
If I had used glass I would have used a sand bath which might have taken ages to reach that temperature
Quote: Originally posted by unionised  | If this is to be believed then the calcium salt is about the worst option
A. Górski, A. D. Kraśnicka. Influence of the cation on the formation of free hydrogen and formaldehyde in the thermal decomposition of formates.
Journal of Thermal Analysis 1987, 32 (5) , 1345-1354. DOI: 10.1007/BF01913334
Fig 1 suggests that zinc formate would be a better choice. (Though their addition of borohydride to the mixture complicates interpretation)
Essentially, if you get carbonate as a product, then that implies that the carbon from the formate was not converted to formaldehyde.
|
Hmmm, that's interesting; the calcium salt seems to require the highest temperature to decompose, so that too would indicate it isn't the best option
I thought one of the formate ions was oxidised to carbonate, the other being reduced to formaldehyde.
I shall try some more zinc formate, see if that works better (I didn't really try the zinc formate on a big enough scale the first time to know for
sure whether it was working properly)
I forgot to mention in my first post, I also tried the formates of magnesium and copper, with no formaldehyde produced
Quote: Originally posted by kmno4  | By the same authors:
Origin of organic gaseous products formed in the thermal decomposition of formates. Journal of Thermal Analysis 32, 1243–1251 (1987)
(DOI:10.1007/BF01905178)
....and from there:
(heating performed under nitrogen) |
Nice! Its odd how they suggest only the lead and tin salts produce formaldehyde, and even then, in pretty low concentrations.
Well, initially I just thought I could easily make it, I tend to like making stuff, just for fun, but now I'm even more determined to get it to work
Does anyone have any experience with formaldehyde gas, especially its solubility?
It is listed at 400g/L, which is even higher than the solubility of ammonia, so theoretically it should dissolve as soon as its brought within a mile
of water, thus I imagine my method of bubbling it through water should be very effective at dissolving it, but maybe there is some other factor
affecting the ease with which it dissolves?
I also did an experiment where I heated the tin formate in a test tube with a tube attached, and bubbled the gas thus produced into an upside down
graduated cylinder filled with water;
The result was a volume that corresponded almost exactly to the volume of co2 that should be produced, and it did test positive for co2, but in other
experiments I ended up with a gas that seemed to have the properties of carbon monoxide, but its pretty hard to test for.
Is formaldehyde solution supposed to smell of formaldehyde?
I still have the solution I made using calcium formate with methane as a carrier gas, and the solution, although apparently the same weight as before
I bubbled the gas through, now has a strong stinging smell, presumably formaldehyde.
Maybe the obvious answer is the right one; perhaps my weight measurements have gone wrong, and in fact I do have a solution of formaldehyde...
I wish there were some other quantitative test of it.
I thought maybe Tollen's reagent, weighing the amount of silver deposited...
Thanks for all your replies!
[Edited on 17-5-2020 by SplendidAcylation]
[Edited on 17-5-2020 by SplendidAcylation]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
"The result was a volume that corresponded almost exactly to the volume of co2 that should be produced, and it did test positive for co2, but in other
experiments I ended up with a gas that seemed to have the properties of carbon monoxide, but its pretty hard to test for."
I believe that you can absorb CO into acidified (with HCl) cuprous chloride solution, etc.:
Attachment: gump1930.pdf (422kB)
This file has been downloaded 468 times
Of course, CO2 can be absorbed easily with sodium hydroxide, but this will also destroy any formaldehyde that is present (Cannizzaro Reaction).
[Edited on 20-05-17 by WGTR]
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by WGTR  | "The result was a volume that corresponded almost exactly to the volume of co2 that should be produced, and it did test positive for co2, but in other
experiments I ended up with a gas that seemed to have the properties of carbon monoxide, but its pretty hard to test for."
I believe that you can absorb CO into acidified (with HCl) cuprous chloride solution, etc.:
Of course, CO2 can be absorbed easily with sodium hydroxide, but this will also destroy any formaldehyde that is present (Cannizzaro Reaction).
[Edited on 20-05-17 by WGTR] |
Nice! I might give that a try.
Yes, the dreaded Cannizzaro reaction.
At the moment I'm focusing on zinc formate, as it seems to be better yielding than calcium formate, but much easier to make than tin formate, so it
seems like a good compromise.
Thanks for the suggestion, at least something can absorb CO!
|
|
|
kmno4
International Hazard
    
Posts: 1496
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Another paper about thermal decomposition of formates:
Darstellung von Formaldehyd und Methylalkohol aus Formiaten, by K. A. Hofmann and Helge Schibsted ; Ber. 51, 1398-1418, 1918
(DOI:10.1002/cber.19180510230).
Unfortunately the PDF is not OCRed in German, so machine translation is problematic. It ought to be re-OCRed in German language, but it is a job for
interested (I am not)
However.... re-OCRed version
Attachment: hofmann1918.pdf (382kB)
This file has been downloaded 388 times
[Edited on 19-5-2020 by kmno4]
Слава Україні !
Героям слава !
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  | "The result was a volume that corresponded almost exactly to the volume of co2 that should be produced, and it did test positive for co2, but in other
experiments I ended up with a gas that seemed to have the properties of carbon monoxide, but its pretty hard to test for."
I believe that you can absorb CO into acidified (with HCl) cuprous chloride solution, etc.:
Of course, CO2 can be absorbed easily with sodium hydroxide, but this will also destroy any formaldehyde that is present (Cannizzaro Reaction).
[Edited on 20-05-17 by WGTR] |
Unless you are keeping your gases very dry, the formaldehyde already dissolved into some condensed water somewhere.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It looks like zinc formate produces methyl formate- which is interesting.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by kmno4  | Another paper about thermal decomposition of formates:
Darstellung von Formaldehyd und Methylalkohol aus Formiaten, by K. A. Hofmann and Helge Schibsted ; Ber. 51, 1398-1418, 1918
(DOI:10.1002/cber.19180510230).
Unfortunately the PDF is not OCRed in German, so machine translation is problematic. It ought to be re-OCRed in German language, but it is a job for
interested (I am not)
However.... re-OCRed version
[Edited on 19-5-2020 by kmno4] |
Wow, there is some amazingly helpful info here, thanks!
Funny story, I spent quite some time today OCRing it and translating it, as I hadn't seen your post edit, haha!
Looks like my idea of using a carrier gas seems to be the way to go, and indeed a 30% yield is very acceptable, I wonder what alternative to asbestos
could be used to hold the powder..
I prepared some zinc formate recently, and proceeded to dry it on the hot plate, in a glass dish
Quite suddenly, the powder melted into a clear syrupy liquid, and proceeded to bubble, with no odour discernible.
Initially it occurred to me that perhaps it was decomposing into carbon monoxide, so I took it off the heat, and eventually it stopped bubbling and
solidified into a hard glassy layer.
Upon further investigation, I found out that zinc formate forms a dihydrate, which is probably what the molten stuff was.
I'll try to carefully dry it without decomposing the anhydrous product.
I translated the Goldschmidt paper, and it seems to suggest a 30% yield of formaldehyde from tin formate, not the quantitative yield often quoted;
Or, rather, the yield is quantitative, but two moles of formaldehyde react forming methyl formate, while one mole is released, mostly in the form of
paraformaldehyde
The Hofmann paper seems to suggest that yields of 30% formaldehyde can be obtained from zinc formate, which makes the zinc salt a better option due to
the ease of preparation.
Interestingly, it says that calcium formate gives very low yields of formaldehyde, so maybe the calcium salt is a non starter after all!
|
|
|
Alkoholvergiftung
Hazard to Others
  
Posts: 174
Registered: 12-7-2018
Member Is Offline
|
|
The patent says if you mix formiate with acidic salts like ironsulfate ore zinksulfate you can push the Reaktion to an yield of 50%. But you lose
space because you need 2.5 times of the acidic salt for this Reaktionl. But it lowers the Temperature.Calciumformiat alone you Need 350C with sulfate
you Need only 250C.
He wrote if you mix it with Silicagel 5%-10% you get 55% Methanol.
Attachment: DE000000316217A_1FormaldehydDestillation.pdf (41kB)
This file has been downloaded 377 times
Attachment: DE000000316217A_2FormaldehydDestillation.pdf (18kB)
This file has been downloaded 352 times
[Edited on 22-5-2020 by Alkoholvergiftung]
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by Alkoholvergiftung  | The patent says if you mix formiate with acidic salts like ironsulfate ore zinksulfate you can push the Reaktion to an yield of 50%. But you lose
space because you need 2.5 times of the acidic salt for this Reaktionl. But it lowers the Temperature.Calciumformiat alone you Need 350C with sulfate
you Need only 250C.
He wrote if you mix it with Silicagel 5%-10% you get 55% Methanol.
[Edited on 22-5-2020 by Alkoholvergiftung] |
Wow, this is interesting, thanks!
I do have some iron sulfate, I'll give that a go.
Some new updates:
I continued to heat the zinc formate, to decompose the dihydrate that I believed I had, the liquid salt turned light brown and continued to produce
bubbles of odorless gas.
Holding wet pH paper above it showed an acidic nature of the gas (I checked to confirm that the salt itself was neutral, so any nebulized salt
wouldn't give a false positive with the pH paper), so I imagine it is CO2.
Holding a cold glass above the bubbling salt resulted in no condensation forming.
So based on these observations I figured that the dihydrate, if it ever existed, had decomposed long ago, and that the salt was now decomposing...
But with no formaldehyde or anything with any smell whatsoever?
I thought that maybe paraformaldehyde was forming, and remaining polymerized, so I took some of the molten sludge and heated it in a test tube; the
result was a lot of flammable gas produced, and a smell of formaldehyde; there was enough gas produced to maintain a continuous flame at the end of
the test tube!
There was a lot more gas produced here than when I heated the precipitated, gently dried, zinc formate; seemingly heating the salt until it melted and
lost co2 had helped.
So I put it back on the hot plate and continued to heat it until I was left with a brown solid, which I hopefully thought might be a mixture of ZnO
and formaldehyde... But no, it seems to be almost exclusively zinc oxide!
I'm planning to try an organic solvent extraction to extract any paraformaldehyde that might be there, but it doesn't look hopeful 
|
|
|
|