| Pages:
1
2 |
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Use For Menthol?
Hello everyone, I have about a half kilo or so of menthol, so I was wondering if there is
anything interesting I can do with it.
I know it functions like a secondary alcohol, but are any of its compounds interesting?
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
unionised
International Hazard
    
Posts: 5129
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I sometimes wondered if it could be used like camphor in Rast's method for measuring molar masses.
[Edited on 19-4-20 by unionised]
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
Make some sodium. https://youtu.be/BsNoiFj3wlw
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Yeah, I saw that awhile back. I found a post here that is just distilling a mixture of NaOH and Al, if that didn't work I was going to do it
NurdRage's way.
EDIT: Found it http://www.sciencemadness.org/talk/viewthread.php?tid=82406
[Edited on 19-4-2020 by Kobold vor NH4]
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
If sodium metal isn't interesting enough menthol valerate is a tranquilizer of some sort
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
That's a pretty smart setup for home distillation of sodium. I was thinking about how I could purify sodium in a home setting, other than the stirring
in dioxane method.
edit: Just thinking out loud, I wonder how well one of those Chinese induction heaters from eBay would work to heat up a CO2 canister containing
sodium?
[Edited on 4-19-2020 by monolithic]
|
|
|
Fery
International Hazard
    
Posts: 1029
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
draculic acid69 thx for interesting idea, it seems to be menthyl isovalerate, I will certainly try the synthesis in the future
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2801
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You can eliminate the alcohol with dilute H2SO4 to 3-menthene. There is probably a way to convert this to the tertiary hydroperoxide, which would
function as a (sort of) chiral oxidant and is an OTC analog of tBuOOH.
[Edited on 19-4-2020 by clearly_not_atara]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
draculic acid69 its not that metallic sodium isn't interesting, I just prefer the other method.
Clearly_not_atara, if the 3-menthene is an alkene, it could be (eventually) turned into a Carboxylic acid, which could could then be esterified with
menthol, right? Could be interesting. Anyone know if these would be possible/stable?
Alcohol->Alkene->Haloalkane->Nitrile->Carboxylic acid->Ester
[Edited on 20-4-2020 by Kobold vor NH4]
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
DraconicAcid
International Hazard
    
Posts: 4360
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Kobold vor NH4  | draculic acid69 its not that metallic sodium isn't interesting, I just prefer the other method.
Clearly_not_atara, if the 3-menthene is an alkene, it could be (eventually) turned into a Carboxylic acid, which could could then be esterified with
menthol, right? Could be interesting. Anyone know if these would be possible/stable?
Alcohol->Alkene->Haloalkene->Nitrile->Carboxylic acid->Ester |
That should read Alkene -> Haloalkane, but yes, that should be possible.
You could also (theoretically) take the haloalkane, react it with magnesium to get the Grignard, add carbon dioxide to get the carboxylate. Beats
mucking with cyanide.
Making esters of menthol won't be very easy, as it's a secondary alcohol.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by Kobold vor NH4  | draculic acid69 its not that metallic sodium isn't interesting, I just prefer the other method.
Clearly_not_atara, if the 3-menthene is an alkene, it could be (eventually) turned into a Carboxylic acid, which could could then be esterified with
menthol, right? Could be interesting. Anyone know if these would be possible/stable?
Alcohol->Alkene->Haloalkane->Nitrile->Carboxylic acid->Ester
[Edited on 20-4-2020 by Kobold vor NH4] |
That would make an interesting ester
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by Kobold vor NH4  | draculic acid69 its not that metallic sodium isn't interesting, I just prefer the other method.
Clearly_not_atara, if the 3-menthene is an alkene, it could be (eventually) turned into a Carboxylic acid, which could could then be esterified with
menthol, right? Could be interesting. Anyone know if these would be possible/stable?
Alcohol->Alkene->Haloalkene->Nitrile->Carboxylic acid->Ester |
That should read Alkene -> Haloalkane, but yes, that should be possible.
You could also (theoretically) take the haloalkane, react it with magnesium to get the Grignard, add carbon dioxide to get the carboxylate. Beats
mucking with cyanide.
Making esters of menthol won't be very easy, as it's a secondary alcohol. |
Secondary alcohols can form esters fine, through Fischer esterification. But reaction is slow because of steric hindrance. Making isopropyl acetate
requires 18 hour reflux.
Theres no need to go from alcohol to alkene to haloalkane. Secondary Alcohols can be directly converted to alkyl bromides without rearrangement with
phosphorus tribromide, but hazmat shipping is expensive. I have never done hydrohalogenation of an alkene, dunno how easy it is.
this would be a fun project

Edit: the fischer esterification step would be done in toluene, with TsOH generated in situation when sulfuric acid is added
[Edited on 20-4-2020 by Cou]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Cou, that's a nice diagram, however it seems to me that PBr3 is out of my ability to handle safely, regardless of of its availability or cost or
usefulness. Phosphorus(and Phosphine) is one of those things that I refuse to work with, as working with it seems what I would call "Hard-Core". Maybe
sometime in the future.
Here's a diagram that I made, which is probably what I will do when my order of Magnesium arrives, sometime next month. Let me know if there's
anything that I missed/got wrong. Ill do an update on it when I attempt the synthesis.
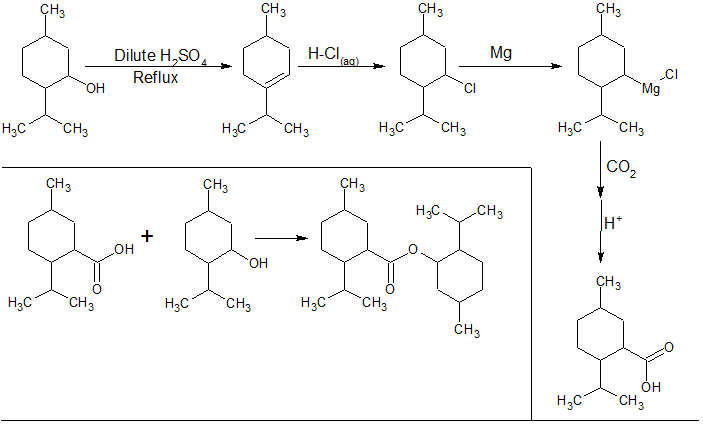
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
You don't need to go through the steps of dehydration alcohol to alkene, then hydrohalogenation. secondary alcohols can be directly converted to a
racemic alkyl bromide by adding to azeotropic hydrobromic acid. the procedure would be similar to nurdrage's synthesis of 1-bromohexane: https://www.youtube.com/watch?v=Ydn1D4FSqkc . Alkyl bromides are easier to turn into grignard reagents than alkyl chlorides, and also easier to
form b/c bromide ions are better nucleophiles, whereas making alkyl chlorides requires zinc chloride catalyst.
One difference between using PBr3 vs HBr to make alkyl bromides from alcohols is that PBr2 only reacts SN2 so that stereochemistry is inverted (the
carbon atom containing the alcohol in menthol is chiral), whereas HBr reacts SN1 so a racemic mixture is made.
I thought dehydration of alcohols to alkenes is done in concentrated sulfuric acid, not dilute.
Vogel's practical organic chemistry doesn't have any procedures for hydrohalogenation of alkenes x(
also, wouldn't the hydrochlorination be markovnikov addition, so the chlorine is added to the tertiary carbon?
here is a revised diagram
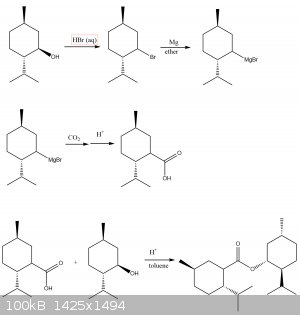
[Edited on 21-4-2020 by Cou]
[Edited on 21-4-2020 by Cou]
|
|
|
Boffis
International Hazard
    
Posts: 1886
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Could you de-hydrogenate the central cyclohexane ring to a benzene ring to give you thymol or oxidize it directly to thymoquinone?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
You could do it if you know how to build a fixed bed reactor. http://translationportal.epo.org/emtp/translate/?ACTION=desc...
|
|
|
Texium
Administrator
       
Posts: 4623
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Quote: Originally posted by Cou  | | I thought dehydration of alcohols to alkenes is done in concentrated sulfuric acid, not dilute. |
It depends
on the alcohol. According to Wikipedia, “ Menthol is easily dehydrated to give mainly 3-menthene, by the action of 2% sulfuric acid.” So it sounds
like menthol dehydrates very readily.
Quote: Originally posted by Cou  | | Vogel's practical organic chemistry doesn't have any procedures for hydrohalogenation of alkenes x( |
It
shouldn’t be too complicated. Dissolving the alkene in an aprotic solvent and bubbling in HCl or HBr has from a simple salt/sulfuric acid setup with
drying trap should do the job perfectly.
Quote: Originally posted by Cou  | | also, wouldn't the hydrochlorination be markovnikov addition, so the chlorine is added to the tertiary carbon |
Yes, you’re correct. If he wanted the anti-markovnikov addition, he would need to use HBr with a catalytic amount of peroxide to
set off a free-radical chain reaction. Overall, skipping the dehydration and going directly to the halide is much more practical.
Side note: it’s nice to see you being productive and helpful, Cou. Your new signature is very large though.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I just remembered that HBr substitution on secondary carbons can cause rearrangement, so the bromine may end up on the tertiary carbon. That is why I
originally suggested PBr3
This means you need to either use PBr3 (Stereochemistry inversion), or dehydrate to the alkene and do anti-markovnikov addition of HBr to the alkene
(racemic)
[Edited on 22-4-2020 by Cou]
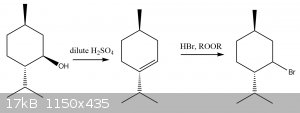
correction on that diagram: the bond connecting the cyclohexane ring to the isopropyl group should be a straight line for the 2nd two molecules, not
pointing into the page
[Edited on 22-4-2020 by Cou]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Here's a file that I found the other night for the dehydration http://jan.ucc.nau.edu/~jkn/235L5-Dehydration.htm . Ill revise my diagram on my previous post either tonight or tomorrow.
I haven't got any Bromides or HBr, so Ill have to order some.
[Edited on 22-4-2020 by Kobold vor NH4]
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2801
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Kobold vor NH4  | Clearly_not_atara, if the 3-menthene is an alkene, it could be (eventually) turned into a Carboxylic acid, which could could then be esterified with
menthol, right? Could be interesting. Anyone know if these would be possible/stable?
Alcohol->Alkene->Haloalkane->Nitrile->Carboxylic acid->Ester |
You don't need to go through the alcohol to make the bromide and you definitely don't want to mess with cyanide or Grignards at the level you're at
right now.
If you want to make a carboxylic acid a better way would be to cleave the alkene with KMnO4. That's only two steps.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
But where would the carbon that's in the carboxylic group come from? Without cutting it open, I do not see how a carboxylic could be formed.
I've mainly studied and worked with inorganic compounds, I have experience working with hazardous compounds, I have the safety mindset, there's just
some things that I don't like.
EDIT:
I haven't worked with cyanides for the aforementioned reasons. I haven't worked with Grignards, Organics or Organometallics because my focus has
mainly been on mineral-bearing rocks.
[Edited on 23-4-2020 by Kobold vor NH4]
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
Texium
Administrator
       
Posts: 4623
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
KMnO4 will indeed “cut open” the alkene under acidic conditions at high temperature. What you end up with is a carboxylic acid on the
formerly secondary carbon of the alkene, and a ketone on the formerly tertiary carbon.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2801
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by Kobold vor NH4  | | I've mainly studied and worked with inorganic compounds, I have experience working with hazardous compounds, I have the safety mindset
|
Could you provide some examples? So far you haven't heard of many reactions that I usually think of as elementary. Cyanide tends to dissuade even
relatively experienced members of this forum. Grignards usually require air-free techniques that are not common in inorganic chemistry.
[Edited on 22-4-2020 by clearly_not_atara]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by Texium (zts16)  | | KMnO4 will indeed “cut open” the alkene under acidic conditions at high temperature. What you end up with is a carboxylic acid on the
formerly secondary carbon of the alkene, and a ketone on the formerly tertiary carbon. |
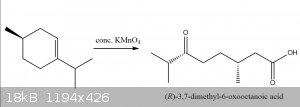
correction version of the diagram i posted above, the stereochemistry was wrong
[Edited on 22-4-2020 by Cou]
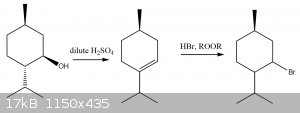
[Edited on 22-4-2020 by Cou]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4360
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Cou  | Quote: Originally posted by Texium (zts16)  | | KMnO4 will indeed “cut open” the alkene under acidic conditions at high temperature. What you end up with is a carboxylic acid on the
formerly secondary carbon of the alkene, and a ketone on the formerly tertiary carbon. |
correction version of the diagram i posted above, the stereochemistry was wrong
[Edited on 22-4-2020 by Cou]
[Edited on 22-4-2020 by Cou] |
You should have a straight line on the carbonyl, not a dashed wedge.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2 |