Saponification of ethyl hydroferulate to hydroferulic acid
In thread
http://www.sciencemadness.org/talk/viewthread.php?tid=154761
I described the reduction of the double bond in a ferulic acid ester with NiCl2/Zn, but wasn't able to definitively characterise the
resulting oily liquid.
I've since used NaOH to saponify the ester and confirmed that the resulting carboxylic acid has the appropriate melting point.
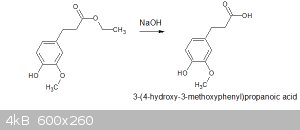
4.26g of dihydroferulic acid was suspended in 50ml of 1M NaOH and refluxed for 1.5hrs. The resulting clear(-ish) solution was allowed to cool to RT,
then acidified carefully with 3M HCl to a pH of 3 - 4.
The volume of liquid was then reduced to about 1/4 of the original volume using low heat, and then allowed to cool. An oil separated, that over a
period of several days crystalized.
The product was washed, then re-crystalized twice from hot (not boiling) water, resulting in 1.2g of a buff coloured, slightly crystalline product.
Measured MP 88 - 89C, (lit 88 - 89C).
My starting material was quite discoloured, so likely had a fair amount of impurity. Hence my yield was low at around 33%, but not bad (I thought) for
a first attempt.
|