Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making Manganese Iodide MnI2
Continuing the quest to make a pink compound (on request of my daughter!) I had a go at Manganese iodide.
I first tried reacting Mn powder with I2 in Xylene (as I recently made Antimony Iodide) but after 90 minutes nothing seemed to have happened. Poormans
Chemist then suggested I try in water. And this worked.
- 100ml water in 500ml beaker
- 2g Mn powder
- 5g I2 granules (or should I say crystals??)
The reaction started even before I started to stir or heat up the water!
- Stirred at high speed and heated to around 50 deg C. Solution was initially dark grey / almost black but turned dark brown after 8 minutes.
- Decided to add more now that it appeared to be doing something. Added 6g of Iodine first; some purple Iodine vapour observed. Added another 3,3g
Manganese powder, Iodine vapor stopped almost immediately and after a few minutes solution turned dark brown again.
- Stirred for another 10 minutes. Increased the temperature to near boiling to see if any more free Iodine was present but no vapors.
- Filtered the hot solution. Filtrate was clear, like water.
- Started to boil down and also tested a few ml of the clear filtrate in a test tube with a solution of lead nitrate. A bright yellow precipitate of
lead iodide, happy days!!
- Solution color became more yellow-pinkish as it was boiled down. Volume less then 10ml when some crystal film started to form.
- Transferred to an ice bath. It very quickly solidified in to a pink layer that was as hard as a rock! Heated up again till liquid and poured onto a
mortar. Agitated with a stainless spatula while it cooled down but it again became rock hard. Used a big flat screw driver and brute force to break up
the MnI2, Transferred to a small vial.
- Final recovery 9.6 gram.
The MnI2 is hygroscopic. Small pieces around the bench that spilled during the breaking up each became wet after some time.
If anyone can advise a technique to use when working with salts that dry rock hard to avoid the struggle of having to get it out from the drying dish
please share.
By the way I did search all forums for "Manganese Iodide" and MnI2 to see if I could post this into an existing thread but nothing came up.
-
   
|
|
|
woelen
Super Administrator
        
Posts: 8015
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Nice synthesis. Given the pink color of your product, you also succeeded in getting a product of decent purity.
Most likely this is not MnI2, but some hydrate of it, MnI2.nH2O (I don't know n).
The color of this compound is darker than that of MnCl2.4H2O, but this is quite common with colored iodides.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
What about the solubility of MnI2 in for example ethanol or acetone? Maybe you get a more fine precipitate when you crash out the salt with a
solvent... Or you get a two layer system, also possible.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Woelen when the initial yield became rock hard and stuck in the beaker I heated it up again until liquid and boiled a bit more before pouring it out
into the mortar so for sure the water is now an in-between number.
Tsjerk it did not work in xylene; I do not know if it will work in other solvents. When I first tried to make tin iodide I tried in acetone but that
did not work. It seemed the iodine had some reaction with the acetone and when the orange solution was heated to evaporate the acetone some iodine
always appeared and messed up the product.
I want to stay with making iodides for now, planning to do lead, erbium, gallium, chromium, bismuth, vanadium, and indium as I get hold of the metals.
They are all said to yield colorful iodides. If you know of any other colorful iodides please tell me 
|
|
|
j_sum1
Administrator
       
Posts: 6327
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Cool project and nice result.
IIRC, cobalt iodide is also quite pretty.
I have some PbI2 ready to process - beautiful deep yellow colour with a slight sparkle.
I may have to try the MnI2.
Please continue to post progress on these.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
@j_sum1 will do. I made lead iodide some time ago to do the golden rain experiment and want to make more just for a sample. The antimony tri iodide is
also very pretty, I posted some photos a few days ago. Red with sparkles.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Lion850  |
Tsjerk it did not work in xylene; I do not know if it will work in other solvents. When I first tried to make tin iodide I tried in acetone but that
did not work. It seemed the iodine had some reaction with the acetone and when the orange solution was heated to evaporate the acetone some iodine
always appeared and messed up the product.
|
What I meant was adding ethanol or acetone to the colorless (aqueous) post reaction filtratie, instead of boiling it down.
|
|
|
woelen
Super Administrator
        
Posts: 8015
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you want a really beautiful iodide, try HgI2. This is the most brilliant, deeply saturated orange I have ever seen, especially if you make the dry
and pure compound.
I made a small sample of that and kept this around. It also has a special property. If heated, it changes color to bright yellow, like powdered PbI2
and on cooling down, it remains yellow, but as soon as it is mechanically agitated (e.g. by shaking), it becomes bright orange again.
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Nice reaction and love the hue of the product - as mentioned above it looks like you got a pretty pure product out of it.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The reaction of zinc with iodine in methanol works fine- it may also be a suitable solvent for reaction with other metals.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sulaiman
International Hazard
    
Posts: 3700
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Lion850  | If anyone can advise a technique to use when working with salts that dry rock hard to avoid the struggle of having to get it out from the drying dish
please share.
|
Rather than crashing out crystals,
why not let the salt crystalise slowly by room temperature evaporation of the water ?
(start with the solution slightly less than saturated at RT)
this way you get larger, purer crystals - eventually.
Depending upon conditions it could take several days,
and your daughter can watch the crystals growing day by day.
This method will normally produce the fully hydrated form.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Sulaiman  | Quote: Originally posted by Lion850  | If anyone can advise a technique to use when working with salts that dry rock hard to avoid the struggle of having to get it out from the drying dish
please share.
|
Rather than crashing out crystals,
why not let the salt crystalise slowly by room temperature evaporation of the water ?
(start with the solution slightly less than saturated at RT)
this way you get larger, purer crystals - eventually.
Depending upon conditions it could take several days,
and your daughter can watch the crystals growing day by day.
This method will normally produce the fully hydrated form. |
I guess you need pretty dry air to do this, as hygroscopic properties were mentioned, but in a box with some CaCl2 this should work.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Woelen - by coincidence today I got some LR quality mercury iodide - and it is a beautiful bright red. I took one look and closed it again, a bit
worried because of the reported toxicity.
Tsjerk - sounds good, but the manganese iodide seems very hygroscopic so it will probably have to be dried enclosed in a container with something like
concentrated sulphuric acid or maybe calcium chloride?
Thanks for all the suggestions.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
At a reasonable price you can buy polycarbonate dessicators these days. Something you might consider. You'll need a vacuum pump also. I once obtained
a thick glass dessicator and I know one thing for sure: I should have gotten one much earlier.
I'd be interested in your Erbium iodide, if you succeed in making it. I once tried for NdI3, which is said to be green(!) in anhydrous form, but
metathesis is a hard thing for NdI3. (Maybe with CuI or AgI a doable metathesis exists?)
Further to pink compounds, I can advise you take some Cr(III) solution and add a slight excess of ammonium paramolybdate, (NH4)6Mo7O24, which goes
through a spectacular series of colour changes and ultimately crystallises as deep pink crystals of (NH4)6[Cr(MoO4)6].
Bisethylenediamine nickel(II) is also bright pink-purple. Make sure to create the bis complex, because the mono complex is blue and the tris complex
is dark purple.
Cr(III) hexamolybdate 
bis ethylenediamine Ni(II) 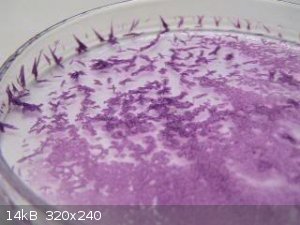
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The end point equilibrium (water content in compound A vs. compound B) will be the same whether you use a vacuum or not, the vacuum will speed up
things though.
Calcium chloride in a closed container will dry a lot of things.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Bezaleel thanks for the suggestions re the pink compounds.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nickel dimethylglyoximate is pink, as is bis(ethylenbnediamine)carbonatocobalt(III) nitrate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8015
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@Bezaleel: The bis(ethylenediamine) nickel(II) complex is dark blue, not pink. I never succeeded in separating this complex from solution though, I
tried a few times, with different anions (sulfate, perchlorate, and chloride).
You can nicely see the different colors of the nickel-ethylenediamine complexes by adding drops of a 30% solution of ethylenediamine to a few ml of a
solution of nickel sulfate.
You first get a blue color, like the color of a solution of CuSO4. After a few drops, the color changes, the color shifts to a deep royal blue, quite
similar to the well-known deep blue copper(II)-ammonia complex. After several more drops, the color shifts to purple.
The sulfate and perchlorate of the tris-complex crystallize very well. They are easy to obtain in a pure and crystalline form, from a solution which
has a slight excess of ethylenediamine.
The bis-complex does not crystallize. On standing in contact with air you get a dirty, ugly-looking dark brown sticky paste, which does not dry
further.
I never tried to isolate the mono-complex. Making that pure is not easy either I think, you must add exactly the right amount of ethylenediamine.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per an MSDS on MnI2 at https://www.ltschem.com/msds/MnI2.pdf:
"Incompatible Conditions: Air, Water/moisture, Oxidizing Agents, Light, Acids "
I believe that MnI2 in the presence of moisture and air/O2 for the transition metal Mn, like for many other transition metals (including iron),
engages in the electrochemical redox reaction:
4 Mn(ll)/Fe(ll) + O2 + 2 H+ --> 4 Mn(lll)/Fe(lll) + 2 OH- (see https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
where the H+ is sourced from water. More details on Mn-oxo species at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6061251/ .
Apparently, a reaction of the unstable the Mn(lll) species moving to Mn(lV) (and Mn(ll)) is further possible resulting in MnO2.
[Edited on 29-11-2019 by AJKOER]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4339
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'd just like to add that the oxalate of the tris complex also crystallizes beautifully.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
@Bezaleel: You wrote "I can advise you take some Cr(III) solution and add a slight excess of ammonium paramolybdate, (NH4)6Mo7O24, which goes through
a spectacular series of colour changes and ultimately crystallises as deep pink crystals of (NH4)6[Cr(MoO4)6]."
I have a bit of ammonium paramolybdate as well as Cr (iii) nitrate and want to make the (NH4)6[Cr(MoO4)6] you mention. I tried to find a more detailed
procedure online but no luck. Can you please give me some idea in terms of grams how much of each of the chromium nitrate and ammonium paramolybdate
to use? Thanks in advance.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by woelen  | @Bezaleel: The bis(ethylenediamine) nickel(II) complex is dark blue, not pink. I never succeeded in separating this complex from solution though, I
tried a few times, with different anions (sulfate, perchlorate, and chloride).
(...) |
I took this from the picture description I added when I made it. I also had in mind that it's the tris complex which is pink, as you mention. You must
be right, but I will also look it up in my logbook.
Quote: Originally posted by Lion850  | @Bezaleel: You wrote "I can advise you take some Cr(III) solution and add a slight excess of ammonium paramolybdate, (NH4)6Mo7O24, which goes through
a spectacular series of colour changes and ultimately crystallises as deep pink crystals of (NH4)6[Cr(MoO4)6]."
I have a bit of ammonium paramolybdate as well as Cr (iii) nitrate and want to make the (NH4)6[Cr(MoO4)6] you mention. I tried to find a more detailed
procedure online but no luck. Can you please give me some idea in terms of grams how much of each of the chromium nitrate and ammonium paramolybdate
to use? Thanks in advance. |
Lion850, you will need a little more than the stoichiometric amount (so that you will be assured that the reaction completes). I worked from chrome
alum, so I don't know how much Cr(NO3)3 you need. You can calculate it from the reaction equation though:
6 (NH4)6Mo7O24(aq) + 7 KCr(SO4)2(aq) + 24 H2O --> 7 (NH4)3H6[Cr(MoO4)6](aq) + 3½ K2SO4(aq) + 7½ (NH4)2SO4(aq) + 3 H2SO4(aq)
You can find the original post here.
Note: (NH4)6Mo7O24.4H2O (1235.86 gram/mole)
If you use too little paramolybdate, then some Cr3+ will remain in solution and the pink complex will be a little darker in colour, because some
uncomplexed Cr3+ will enter into the crystal lattice.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Bezaleel I gave it a go but with unexpected results. But let me immediately add that I did not follow the procedure as per the link you supplied all
that well:
-The app I use for stoichiometry did not accept the equation so I just took a stab as a first try: I dissolved 3g of (NH4)6Mo7O24 in 60ml water and 2g
of Cr(NO3)3.9H2O in 50ml water. Note this was at room temperature which is my shed was 32C yesterday.
- Mixed the solutions. Color briefly went lighter green then very dark green again.
- Waited some 20 minutes while stirring but did not see a color change to grey.
- Heated to 70 for a while but the color more or less stayed very dark.
- Cooled down to room temp (32), still very dark.
- Boiled off until signs of precipitate appeared
- Cooled and filtered.
- Left on the filter paper was a light green powder, this dried quickly at room temperature. Dry light green powder was 1.9 gram.
- The filtrate was a beautiful dark green. This quickly semi-dried to a dark green sticky paste but did not dry any further overnight. Placed on watch
glass on boiling beaker the next morning and ended up with 1 gram of dry very dark green product. I wonder if this is just unreacted Cr(NO3)3, seems a
similar color.
What I find interesting is that the post you reference also says a apple green precipitate resulted, but that others were not able to replicate this
color (if I understand the Dutch correct); but it seems mine went the apple green way.
Any thoughts what the light green and the dark green compounds can be? I also half expected to smell acid or maybe see nitrous oxide fumes during the
boiling off but nothing else was obvious apart from steam.

[Edited on 5-12-2019 by Lion850]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Hi Lion850, what seems to have happened is that you used an excess of Cr3+. The ratio Cr : Mo = 1 : 6 in the final product. Excess molybdate or Cr3+
stay in solution. I never tested properly what happens when you use different ratios, but I obtained the deep green solution also, when I used too
little heptamolybdate.
Taking a molar ratio of 6 heptamolybdate (dihydrate) to 7 chromium, you will need 2.65 g of heptamolybdate for each gram of chromium(III)nitrate
nonahydrate. (Molar masses are 1235.86 and 400.21, the latter taken from the English Wiki.)
One thing to note is that if you heat the solution, and Cr is not longer present as Cr3+(aq), it has a tendency to passivate (at least, for this
particular complexation reaction). This may explain the green powder you obtained (probably Cr2O3 or a hydrate). I suspect that this is also what was
obtained in the early publication and what the authors named "apple green", although I would not personally call this colour apple green.
|
|
|