UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Cobalt (III) Ammine/Chloro complexes and Crystal Field Theory
Depending on what kind of background you have, you may or may not have experience with what is called crystal field theory, which attempts to explain
the colors of transition metal compounds. Essentially, the model states that these colors are the result of excitation of d-orbital electrons. Those
not interested in theory can skip to the syntheses below.
The 5 d-orbitals in an atom have certain spatial arrangements, just as the ligands surrounding a complex have certain spatial arrangements. What
allows a ligand to be a ligand are lone pairs of electrons. If a ligand is closely situated to an orbital, repulsion between the electrons in the
orbital and those in the ligand occur. This repulsion makes that orbital effectively a higher energy state than the other orbitals. The basic
structure the model deals with is an octahedral complex with uniform ligands although it can account for linear, square planar, and tetrahedral
complexes with mixed ligands. Even like this, two of the five orbitals are higher energy than the others. Different ligands cause different levels of
repulsion and thus alter the size of the gap in energy between the two possible energy levels. When light strikes a complex, an electron can absorb a
photon with just enough energy to carry it to the higher energy orbitals. Since photon energy is dependant on the wavelength, and wavelength is
percieved as color, different ligands cause different colors to be absorbed, resulting in the complementary colors to the absorbed color being
percieved as the color of the complex. For example, hexaaquacopper (II) is the familiar blue of aqueous Cu (II). In order to be blue, the complex must
absorb wavelengths in the yellow/orange/red region. These are shorter wavelengths and thus lower energy. The "splitting" between the orbitals must be
fairly small and H2O ligands produce a fairly weak "crystal field" If we add a chloride, we have a color shift to green. Green is allowing blues,
greens, and yellows to pass and only absorbing the very low energy reds and oranges. Cl- produces an even weaker crystal field than H2O.
Information on this ordering can be found as a "spectrochemical series" and the model is considerably more in depth than the summary of the
interesting parts I just paraphrased.
At any rate, we had a very interesting lab to go along with this concept. We synthesized two cobalt (III) complexes and did titrations as well as
optical spectroscopy to help us determine their identity. I will post the syntheses here. The procedure calls for "concentrated hydrochloric acid" and
"concentrated ammonia" I believe these were no different than our usual definitions of these reagents (I will check tomorrow).
Complex 1: Hexaamminecobalt(III) Chloride
1. In 10ml of hot deionized water in a 125ml Erlenmeyer (a beaker will work fine, just cover with a watchglass or something to slow heat dissipation),
dissolve 9.0g of cobalt (II) chloride hexahydrate and 6.0g ammonium chloride.
2.Once all solids are dissolved and the solution is hot, remove from heat and add about 1g of powdered activated charcoal and 20ml of concentrated
aqueous ammonia. Chill the solution under running water followed by an ice bath.
3.To this cold solution, very slowly add, with stirring, 20ml of 6% H2O2. It has a tendency to foam a lot here, so don't rush it.
4. Heat the mixture to 60C on a hotplate and maintain that temperature for 15-20 minutes until the pink color of the solution vanishes and it becomes
golden orange. Some solid may or may not precipitate at this stage. Chill the solution again in an icebath, then filter the solid with a buchner
funnel. Dispose of the filtrate.
5. In a beaker, bring a solution of about 3ml of concentrated HCl in 80ml of deinonized water to a boil. Set up your buchner again and be prepared to
filter. Add the solid from the previous step to the boiling dilute acid and allow the orange material to dissolve away from the activated charcoal.
Quickly filter the hot solution and transfer the filtrate to a clean beaker. The solid collected should be all black and should ideally only be
charcoal although small losses of the product here are common.
6. Chill the solution in an ice bath for a reasonable amount of time. The complex seperates as fine, golden orange needles. Filter through the buchner
one last time and draw air over the product to help dry it, then place in a dessicator to finish drying.
Complex 2: Pentaamminechlorocobalt (III) Chloride
1. Dissolve 5.0g CoCO3 in 15ml of concentrated HCl
2. Add 35 ml deinonized water and gravity filter to remove any insoluble impurities (put the filtrate into a 500ml erlenmeyer)
3. Add 5g ammonium chloride and 50ml concentrated ammonia solution. Chill the solution under cold running water and then an ice bath.
4. To this cold solution, add very slowly, and with stirring, 80ml of 6% H2O2
5. Add conc. HCl to this solution to neutralize it, testing periodically with pH paper. After reaching neutrality, add another 20ml of conc. HCl.
6. Heat the resulting suspension on a hotplate for an hour. A powdery purple precipitate seperates during this time.
7. Cool the solution, then filter off the solid with a buchner. Wash with a few mls of ice water, followed by a few mls of ice cold alcohol. Draw air
over the product to help dry, then place in the dessicator.
These complexes illustrate crystal field theory very nicely in that replacing an ammine with a chloro results in a dramatic color shift. To be orange,
the first complex must be absorbing the high energy blue and purple wavelengths. The "splitting" caused by ammonia as a ligand must be large.
Replacing just a single ammonia with a chloro results in a purple precipitate, which means it is absorbing the relatively low energy orange and yellow
wavelengths instead of high energy purple and blue.
I will hopefully have pictures of these two complexes up by tomorrow...I have to "borrow" the remaining products from lab tomorrow afternoon.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I know this isn't a terribly interesting topic as it isn't intended to be a springboard for experimentation, yet it is clear evidence supporting a
model. It bridges the gap between theory and reality which is not done in the classroom as much as it should be. As promised, pictures...the color is
somewhat off....I tried my best to match it. The pentaamminechlorocobalt(III) chloride is more of a purple-magenta...rosy-violet (its very hard to
assign a name) than the simple "purple" I named it in the above post.
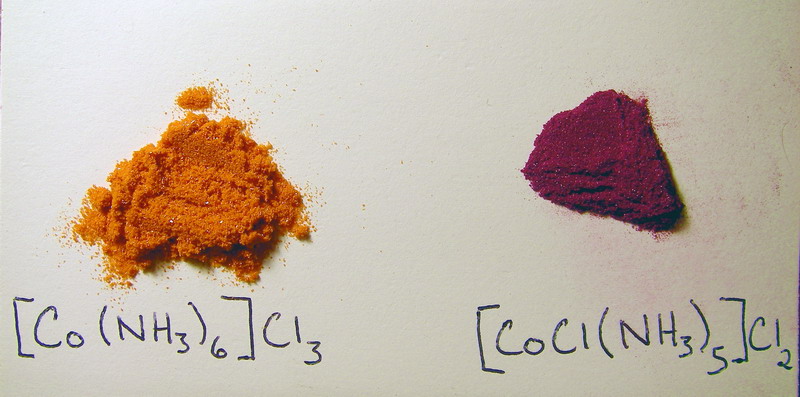
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
That kind of like the dark reddish purpley color Fe2O3 sometimes attains?
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The cobalt complexes are interesting, there's a huge number of them. Even with simple ligands you can get a number of different colours. They also
illustrate the role of pH and ligands in controlling oxidation state, the first Co complex I made used powdered charcoal as the catalyst and air,
first bubbled through aqueous ammonia to prevent lose of NH3 from the reaction mix, as the oxidant. Hydrogen peroxide is more convenient than air, but
air is more accessible.
The cobalt complexes are much less frustrating than the nickel ones which often have several forms depending on exact reaction conditions, the stock
market, phase of moon, and general mood of the nickel ion. The cobalt complexes are also often pretty stable, making them easy to isolate.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
My 250g cobalt chloride hexahydrate (10 EUR including shipping) just arrived. I want to make this complex. Is it also possible to do it without
activated charcoal? Or with an other catalyst?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I would buy some willow charcoal from an art shop or activated charcoal from an aquarium shop and simply use that after crushing it finely.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
You should be able to get activated charcoal for a dollar or two. I have plenty since my normal use for it is as decolorizing charcoal (norit). Mine
is just from an aquarium shop and I threw the chunks in a coffee grinder rather than mortar and pestle small batches all the time.
AFAIK, it is *possible* to do without any catalyst, but incredibly slow. I imagine several hours of bubbling air or oxygen through the mix would
behave similarly to H2O2 + charcoal, but it is really inconvenient considering the charcoal is a few EUR more and you'll have it for other things.
Also, when you add the H2O2, the procedure says to do it slowly. Do it really slowly. Half the class got brightly colored foam on their workspace by
rushing.
A note on stability: These have been sitting in little clear glass jars, probably not even very airtight since the original post. There is no visible
decomposition at all.
[Edited on 9-24-08 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
because this is my first time I do this experiment, and do not want to lose too much precious CoCl2.6H2O, I decided to do it on a 4x smaller scale.
I do not have ammoniumchloride  . So I did the following: add 2,8mL 32% HCl to 2,2mL
25% ammonia. Lots of ammoniumchloride is lost (smoke). I evaporated some water and was left with about 3,5mL of water. A lot of ammonium chloride
crystallised out when I was measuring the liquid, and I lost some during the process. I figured out 3,5mL would be enough, as dissolving 1,5g NH4Cl in
2,5mL water would also increase the volume. I added 2 drops of concetrated hydrochloric acid to compensate for the lost NH4Cl. . So I did the following: add 2,8mL 32% HCl to 2,2mL
25% ammonia. Lots of ammoniumchloride is lost (smoke). I evaporated some water and was left with about 3,5mL of water. A lot of ammonium chloride
crystallised out when I was measuring the liquid, and I lost some during the process. I figured out 3,5mL would be enough, as dissolving 1,5g NH4Cl in
2,5mL water would also increase the volume. I added 2 drops of concetrated hydrochloric acid to compensate for the lost NH4Cl.
Next 2,25g of cobalt chloride hexahydrate was weighed and to this the hot solution of NH4Cl was added. While pouring some NH4Cl crystallised out in
the flask where I made the NH4Cl, even though I heated the entire glass. Next 5mL of 25% ammonia was measured out. I used this to dissolve the
crystallised NH4Cl first, and then added the 5mL to the dark blue solution of cobalt.
250mg of activated charcoal were taken and added to the reaction mixture.
Next I prepared 5mL 6% H2O2 (4 parts water and 1 part 30% H2O2). This was added over a period of 2-3 minutes, while keeping a watch glass on the
beaker (inhalation of droplets containing cobalt can cause cancer, as cobalt is a mild carcinogen by inhalation.). Now I left it to stand. I will make
that an hour or even more, as I don't want to be in the lab 20 minutes warming the reaction to 60C (it's cold outside.). And I don't have a
hotplate/oven. Leaving an open flame heating the beaker while I'm not ther eis not going to happen. I will heat if the reaction isnt done in 2 hours.
|
|
|
|