| Pages:
1
2 |
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
I meant if you used plain ammonia on a ketone, you would need a high pH, not if you used some other amine to form the imine.
"A lack of a hydrogen bonded to the nitrogen is not going to pull it out of solution"
Yes, but it may likely help the equilibrium. Another example is condensation of H2S with an aldehyde proceeds when the aldehyde has a very low
solubility in water, for example PhCH=O
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I very much doubt it's going to have much of an effect on the solubility or the final yield. =NR is still going to be a hydrogen bond acceptor.
Anyway, I need to get some sleep - 'night! 
I weep at the sight of flaming acetic anhydride.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
you could make nitro isopropane from AgNO2 and isopropyl iodide, this a convenient, easily performed reaction, especially at the multikg level,
(anhydrous ether, no UV or visble light etc) then you could reduce the nitro to the amine using your zinc dust or maybe some tin and acid, the workup
for this would be so simple, no insoluble crap everywhere at all!!
i should design custom synthesis for living i'm a natural.
|
|
|
Hexagon
Harmless

Posts: 45
Registered: 11-5-2010
Member Is Offline
Mood: Fanf*ckingtastic
|
|
Quote: Originally posted by mario840  | yes but i wonder if this "method" works , amine will form a layer on top (very low density) or what ? if form there will be easy to extract or
separate withouth heating , i'm just asking that's all  |
Yep if iPrOH separates, then iPrNH2 should separate too, better fully saturate the aq. phase with NaCl or something like that thogh. And yes, as long
you want small ammounts of this amine, roundup is the way to go, it is the most widely used herbicide and you can find it easy on any farm supply
store around the world AFAIK.
[Edited on 17-9-2010 by Hexagon]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
The isopropylamine won't separate, it's miscible in water.
I weep at the sight of flaming acetic anhydride.
|
|
|
Hexagon
Harmless

Posts: 45
Registered: 11-5-2010
Member Is Offline
Mood: Fanf*ckingtastic
|
|
True, but isopropanol is miscible in water too and if you saturate the water of that mixture with a salt like NaCl, you'll obtain a biphasic mixture,
azeotropic iPrOH being on the top of it (with just a little bit of salt) and brine at the bottom of it (with just a little bit of isopropanol in it) I
think it's a safe assumption that isopropylamine will behave similar, or at least it'll be extracted by the IPA.
Even ethanol will salt out with potassium carbonate or ammonium sulfate. Hell, why do I like so much the salting out process? 
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
so better add alone NaCl to solution or previous saturated aq. solution NaCl and then add to the reaction mixture or it doesn't matter ? But when i
extract solution with IPA the first will came out isopropylamine (boiling point much lower then IPA) , so i think why waste of IPA just boil to 40 -
50 C in water bath etc. and evaporate. Another question how to dry this amine ? MgSO4 or Na2CO3 will work ?
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
i have also thinking why not isolate glyphosate too , if Roundup is aqueous solution so we need to add 6 M HCl to ph = 3 and we have insoluble in
water glyphosate crystals and very soluble in water isopropylamine*HCl , next step is take over water layer and make whole solution base to pH = 10
(with 3M or 6 M NaOH) and amine SHOULD form upper layer in strong base solution (in neutral it is miscieble with water also acidic) if NOT just
evaporate amine (32 C) .. so we have 2 chemicals from 1 product and Roundup have very strong product containing up to 45% salt  is this method really possible ?? is this method really possible ??
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Hunig's base? The method of Heinzelmann might work quite nicely. Requires hydrogenation, but nowadays that is easily achieved, via the method of
Brown, using a stir-bar.
Heinzelman produces a methyl-di-isopropylamine, rather deftly from methylamine and two moles of ketone. Should be readily adaptable to large scale
synthesis of Hunig's base.
Heinzelman J.Am.Chem. Society 75, 921 (1953)
As I recall, the procedure is given in the above document, though it is possible, it is in a subsequent paper. Sorry, I no longer have the paper on
hand.
[Edited on 25-9-2010 by zed]
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by Nicodem  | | You could try using the method described in the Example 1 of FR971429 (reductive amination of cyclohexanone with NH3, using Zn + NiSO4), but having a
lower NH3 vs. ketone ratio instead to promote the formation of the secondary amine. |
I was unable to locate that patent using Google web search and patent search. Could you provide it for me if you have it?
If I were to prepare monoisopropyl amine, would the method the thread starter proposes work?
I'm thinking of using acetone, water, zinc dust, Ni++ and HgCl2 catalysts, and an ammonium salt in aqueous solution. But I'm not sure if it would
work; if the process calls for free ammonia, a strong base would be needed. However, that would also precipitate the metal salts. I suppose
if an ammonium salt would work, aqueous ammonia would be just as good.
However, this is all speculation based on the thread starter's assumptions and partly on what I know from the description of the patent you mentioned.
Is there anything to it?
Quote: Originally posted by madscientist  | | You're likely aware of this, but aluminum amalgam does the trick. Dab a bit of HgCl2 on aluminum foil and drop it into the solution of acetone and
ammonia. Your house will smell like a dead whale. |
Does this eliminate the need for the nickel catalyst? If this is a simple preparatory procedure, my only concern will be the work-up.
Quote: Originally posted by Eclectic  | | Ideal system would be water, acetone, ammonium chloride, and a dissolving reactive metal, with or without nickel..., scrap aluminum turnings would be
great if it works, but I have zinc dust. I was just asking if anyone has actually DONE it, or has actual prep procedure. |
Quote: Originally posted by madscientist  | | That is not true - I performed a reductive amination on acetone in methanol/water 50/50 with Al/Hg amalgam and the yields were reasonable. This was 7
or 8 years ago so I can't throw out numbers but it does work and it works well. |
Was this the mono- or diisopropylamine? Is the methanol crucial to the reaction, and could ethanol fit as a substitute? Do you remember how you
performed the work-up?
Does anyone have an actual paper on this reaction, and not just anecdotes and patent references? Thanks. 
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Sorry, I don't recall, it's been too long.
solo provided a paper a while ago detailing reductive amination using nothing but 10% NaOH and zinc dust to effect the reduction. Might be worth a try
since the reagents are cheap.
I weep at the sight of flaming acetic anhydride.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Lambda-Eyde  | | I was unable to locate that patent using Google web search and patent search. Could you provide it for me if you have it? |
You should search patents where they are supposed to be and not in secondary sources. The obvious place for a French patent is the European patent
office site.
| Quote: | | I'm thinking of using acetone, water, zinc dust, Ni++ and HgCl2 catalysts, and an ammonium salt in aqueous solution. But I'm not sure if it would
work; if the process calls for free ammonia, a strong base would be needed. However, that would also precipitate the metal salts. I suppose
if an ammonium salt would work, aqueous ammonia would be just as good. |
I don't see what role would HgCl2 have there. The electrons are transferred from Zn to Ni and from there to the imine, so you don't need any
amalgamation of Zn as this would allow direct transfer of electrons from Zn to the organic substrate and this would be counter productive in regard of
imine vs. ketone chemoselectivity.
I also don't understand what do you mean by "precipitate the metal salts" in regard to ammonia. Which metal salt would be precipitated by ammonia and
why? Ni(II) surely would not as ammonia is a good ligand for Ni2+ cations, Zn is metallic, while Hg(II) was never suggested originally anyway.
Besides, ammonium salts of strong acids are nonnucleophilic so they can't form imines until they are neutralized first.
| Quote: | | However, this is all speculation based on the thread starter's assumptions and partly on what I know from the description of the patent you mentioned.
Is there anything to it? |
Yes, don't bother with speculations when you have no reason to do so. Just follow the instructions of the patent. It was tested on cyclohexanone and
it gives amines from it, so it is not worthless.
| Quote: | Quote: Originally posted by madscientist  | | You're likely aware of this, but aluminum amalgam does the trick. Dab a bit of HgCl2 on aluminum foil and drop it into the solution of acetone and
ammonia. Your house will smell like a dead whale. |
Does this eliminate the need for the nickel catalyst? If this is a simple preparatory procedure, my only concern will be the work-up.
|
That is a common oldfashioned reductive amination of ketones. It gives shitty yields when using ammonia instead of the more nucleophilic alkyl amines,
but basically it can work to some degree. So if madscientist says he got reasonable yields, it may just be so.
The work-up will obviously involve a distillation from the postreaction slurry, followed by acidification of the distillate with HCl(aq), rotavap,
basification of the residue with conc. NaOH(aq), phase separation and again distillation, this time with a distillation column.
I don't really understand your question about nickel catalyst. There is no Ni involved here. This is a different reduction system.
| Quote: | Does anyone have an actual paper on this reaction, and not just anecdotes and patent references? Thanks.  |
As far as I know there is something on Ni/Zn couple for reductive aminations in the scientific literature as well, though perhaps there is much less
written about it when compared to the Ni/Al couple. In any case, it is always wise to do a literature search before engaging in any experimental work.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have extracted isoproylamine from Shore-Klear, an aquatic variant of the herbicide Roundup. I selected an aquatic variant vs Roundup as these do
not contain a surfactant. Whether or not this is important I do not know, it just seemed prudent.
I tested my process on 5mL up to the distillation step and it seemed successful so then proceeded to a 100mL batch. My procedure consists of 4 steps:
1. Acidify with ~75 mL of 6N HCl. This causes the insoluble gyphosate to precipitate as a heavy (sp gr 1.7) white solid. The isopropylamine (IPA)
hydrochloride stays dissolved in the clear aqueous supernate.

2. Separate the glyphosate from the IPA HCl by use of a separatory funnel. Recover more IPA HCl from the glyphosate fraction by centrifugation.
Discard the glyphosate.
3. Basify the clear supernate with about 65mL of 6M NaOH. The IPA is now dissolved in the water as a free base. You will know this by the smell.
It is important if not essential that you do this outside or in a good hood because of this smell.
4. Distill over the IPA (bp 33-34C) using a fractionation column. I used broken glass packing as I have seen several references to IPA reacting with
metals. Much to my surprise there is no azeotrope and the distillation is easy with the stillhead thermometer locking on at 33-34C.
Yield = 13.4g, or 19.4mL. %yield = 76.0%. (Basis = 105mL).
My plan was to use stoichiometric amounts of HCl and NaOH, but due to a calculation error I used quite a bit more than that. I suspect that
stoichiometric amounts would have worked just as well. My choice of molarities was arbitrary.
Experimenting with salting out using NaCl did not yield a phase separation of IPA and water. IPA is miscible with water, alcohol, and ether. That is
the reason I went to distillation. I had also read an article in I&EC describing an industrial separation using distillation.
[Edited on 16-3-2012 by Magpie]
[Edited on 16-3-2012 by Magpie]
[Edited on 17-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I thought I should post the results of further experimentation in the isolation of isopropylamine from Shore-Klear (re: previous post).
The basis for my last batch was 200mL of Shore-Klear. A stoichiometric amount of 6M HCl was used. This brought the pH to ~5. The resulting ppt of
glyphosate settled well, better than before. This was easily removed by Buchner vacuum filtration, so no need for any tedious centrifugation. Yield
was about the same, ie, 72%.
The waste byproduct glyphosate could be recovered as a very nice white solid, probably quite pure. Does anyone know of any use for this in mad
science?
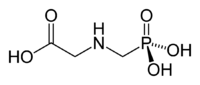
glyphosate
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Dr.Bob
International Hazard
    
Posts: 2734
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
The waste byproduct glyphosate could be recovered as a very nice white solid, probably quite pure. Does anyone know of any use for this in mad
science? |
You could use it to kill some weeds near your science laboratory? :-)
Not much that I can think of to do with it other than that.
|
|
|
Scr0t
Hazard to Others
  
Posts: 118
Registered: 14-1-2012
Location: Europe
Member Is Offline
Mood: Desiccated
|
|
| Quote: | | I have extracted isoproylamine I selected an aquatic variant vs Roundup as these do not contain a surfactant. Whether or not this is important I do
not know, it just seemed prudent. |
The surfactant(s) do not appear to matter. I used Round-up Biactive 360 (more expensive but it's what I had to hand).
I simply distilled a 50% NaOH solution with the Round-up (100% excess of NaOH) and dried the distillate over an equal volume of NaOH before
re-distilling to yield ~80% iPrNH2.
No problems with foaming... pretty straight forward.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Scr0t  |
The surfactant(s) do not appear to matter. I used Round-up Biactive 360 (more expensive but it's what I had to hand).
I simply distilled a 50% NaOH solution with the Round-up (100% excess of NaOH) and dried the distillate over an equal volume of NaOH before
re-distilling to yield ~80% iPrNH2.
No problems with foaming... pretty straight forward. |
I also thought that it might not be necessary to remove the glyphosate first. It's good that you have verified what is likely the most simple method.
What was your yield?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Another simple route to making isopropylamine is the Leuckart–Wallach reaction, where acetone is made to react with ammonium formate.
http://en.wikipedia.org/wiki/Leuckart%E2%80%93Wallach_reacti...
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Shame that I only have ammonium acetate :/
Rest In Pieces!
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Formic acid can be bought for $12 a liter from Dudadiesel. React with standardized ammonia, and you're set!
|
|
|
ciro
Harmless

Posts: 1
Registered: 7-7-2010
Member Is Offline
Mood: No Mood
|
|
Maybe it works by reducing the imine formed by acetone and ammonia with sodium borohydride in acetic acid.
This paper: http://www.erowid.org/archive/rhodium/pdf/nabh4.carboxylic.a... in 2.2. N-Alkylation of amines, describes the alkylaton of aniline to give
N-ethylaniline, but also with acetone and a variation in the conditions (temperature), it gives N-isopropylamine.
[Edited on 26-5-2012 by ciro]
|
|
|
Scr0t
Hazard to Others
  
Posts: 118
Registered: 14-1-2012
Location: Europe
Member Is Offline
Mood: Desiccated
|
|
Diisopropylamine via Isopropylamine alkylation
16.8g iPrNH2 and 43g iPrBr (1.2 eq) in 100ml IPA was refluxed for 36hrs.
Crystals began to form in the hot solution at about 15hrs.
About 25ml of liquid was distilled off removing some left over iPrBr/iPrNH2. After cooling the solids were filtered, rinsed with a little IPA and air
dried to yield Diisopropylammonium bromide as white platelets (61% based on iPrNH2).
Evaporation of the filtrate gave a couple grams more of the product.
Treatment of the salt with strong NaOH solution followed by separation and distillation yields the free-base.
The iPrNH2 used was extracted from Roundup.
The Taiwanese reference given by Entropy51 in
https://www.sciencemadness.org/whisper/viewthread.php?tid=12416&page=2#pid155929
involved reflux for a shorter time (15hrs) in EtOH and obtained a 43% yield.
----------------
Several attempts to use a Zn-Ni couple in a similar fashion to
FR971429 to prepare this secondary amine from Acetone gave no workable amounts even when adding an excess of iPrNH2 into the reaction rather than
in-situ generation with NH3.
I've had no success isolating secondary amines using this reagent.
|
|
|
| Pages:
1
2 |