
starman - 23-12-2011 at 14:23
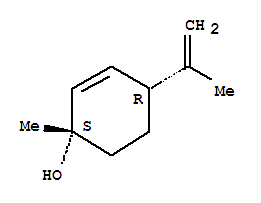
The hydroxyl is directly bound to an unsaturated aromatic ring but the actual carbon it is attached to is fully bound.
Would it be expected to react with dilute aqueous alkali to form a water soluble (phenate) salt?
DJF90 - 23-12-2011 at 14:28
Your ring isn't aromatic, its just an alkene. That happens to be a tertiary allylic alcohol, so no, you won't be able to deprotonate it in a facile
manner.
starman - 23-12-2011 at 17:04
Thanks DJF90.Obviously further study required as to the difference of cyclic alkene and aromatic.
[Edited on 24-12-2011 by starman]
MagicJigPipe - 23-12-2011 at 22:44
Aromaticity requires that there be alternating pi bonds (double bonds) and that there be 4n + 2 pi electrons (two for each double bond) where n is a
positive integer. This ends up giving a pattern of: 2, 6, 10, 14, 18, ... So, for example, benzene is aromatic because it has 6 pi
electrons/orbitals (4*1 + 2 = 6). Cyclobutadiene is anti-aromatic because 4n + 2 can never equal 4. See "Huckel's Rule".
Just look for alternating double bonds in a ring first, then check it against that rule to determine if it's aromatic or not.
Of course, it gets a bit more complicated when you get into larger ring systems but I think this explanation is just fine for now.

