Pyruvic acid
| |
| Names | |
|---|---|
| IUPAC name
2-Oxopropanoic acid
| |
| Preferred IUPAC name
2-Oxopropanoic acid | |
| Systematic IUPAC name
2-Oxopropanoic acid | |
| Other names
Acetylformic acid
Pyroracemic acid α-Ketopropionic acid | |
| Properties | |
| C3H4O3 CH3COCOOH | |
| Molar mass | 88.06 g/mol |
| Appearance | Colorless liquid |
| Odor | Sour, vinegar-like |
| Density | 1.250 g/cm3 (20 °C) 1.267 g/cm3 (25 °C) |
| Melting point | 11.8–12 °C (53.2–53.6 °F; 284.9–285.1 K) |
| Boiling point | 165 °C (329 °F; 438 K) |
| Miscible | |
| Solubility | Reacts with amines, bases Miscible with alcohols, carboxylic acids, ketones |
| Vapor pressure | 1.29 mmHg |
| Acidity (pKa) | 2.50 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Related compounds | |
| Related compounds
|
Lactic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
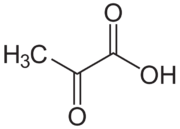
Pyruvic acid is the simplest of the keto acids, as well as alpha-keto acids, which are compounds with a carboxylic acid and a ketone functional group. It has the formula CH3C(=O)COOH.
Pyruvic acid is an important compound in the biological metabolism, by supplying energy to cells through the Krebs cycle.
Contents
[hide]Properties
Chemical
Pyruvic acid is a mid-strength carboxylic acid. It reacts with bases to form salts, called pyruvates.
Pyruvic acid supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking (lactic acid).
Physical
Pyruvic acid is a colorless to slightly yellowish colored liquid, with a sour vinegar-like odor. It is miscible with water and other organic solvents. Due to its melting point of around 11 °C, the pure compound will solidify in cold weather or if placed in a fridge.
Availability
Pyruvic acid is sold by chemical suppliers.
Pyruvates, such as calcium pyruvate, are sold as weight loss supplements. The free acid can be obtained by reacting the pyruvate salt with a strong acid, then distill the free acid under reduced pressure.
Preparation
Pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium bisulfate. The crude distillate is further purified by distilling it under reduced pressure.
Oxidation of lactic acid will produce pyruvic acid. Hydroxyacetone can also be used.
Alternatively, it can be obtained from the oxidation of propylene glycol with a strong oxidizer (e.g., potassium permanganate, sodium hypochlorite/bleach):
- CH3CH(OH)CH2(OH) + [O] → CH3C(=O)COOH
A less common route involves hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:
- CH3COCl + KCN → CH3COCN + KCl
- CH3COCN → CH3C(=O)COOH
Pyruvic acid can be produced from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA.
Projects
- Make pyruvates
- Make lactic acid
Handling
Safety
Pure pyruvic acid is somewhat corrosive and irritant to skin, nose, mouth and eyes. The acid and its salts display little toxicity.
Consumption of excess pyruvates will lead to diarrhea, bloating, gas and increase in low-density lipoprotein (LDL) cholesterol.
Storage
In closed air-tight bottles.
Disposal
No special disposal is required. Discard it was you wish.